Discovery of a Natural Protein that Self-Assembles into Fractal Structures
Core Concepts
A cyanobacterial citrate synthase enzyme self-assembles into Sierpiński triangle fractal structures, demonstrating that intricate and regulatable protein complexes can evolve from non-fractal precursors.
Abstract
The content describes the discovery of a natural protein, citrate synthase from the cyanobacterium Synechococcus elongatus, that self-assembles into Sierpiński triangle fractal structures.
Key highlights:
Fractals are self-similar patterns observed across multiple length scales, but molecular assembly into fractals has been limited to synthetic systems so far.
Using cryo-electron microscopy, the authors reveal how the citrate synthase fractal assembles from a hexameric building block.
Different stimuli can modulate the formation of the fractal complexes, which can also regulate the enzymatic activity of citrate synthase in vitro.
However, the fractal may not serve a physiological function in vivo.
Ancestral sequence reconstruction suggests the citrate synthase fractal may have emerged as a harmless evolutionary accident from non-fractal precursors.
The findings expand the known possibilities for protein complexes and demonstrate that intricate and regulatable assemblies can evolve from a single amino acid substitution.
Emergence of fractal geometries in the evolution of a metabolic enzyme - Nature
Stats
Fractals are patterns that are self-similar across multiple length-scales.
Macroscopic fractals are common in nature, but molecular assembly into fractals has been restricted to synthetic systems.
Quotes
"Here we report the discovery of a natural protein, citrate synthase from the cyanobacterium Synechococcus elongatus, which self-assembles into Sierpiński triangles."
"Although different stimuli modulate the formation of fractal complexes and these complexes can regulate the enzymatic activity of citrate synthase in vitro, the fractal may not serve a physiological function in vivo."
"We use ancestral sequence reconstruction to retrace how the citrate synthase fractal evolved from non-fractal precursors, and the results suggest it may have emerged as a harmless evolutionary accident."
Key Insights Distilled From
by Franziska L.... at www.nature.com 04-10-2024
https://www.nature.com/articles/s41586-024-07287-2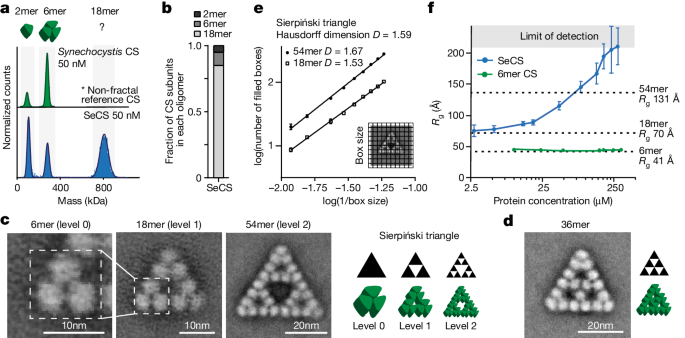
Deeper Inquiries
What other natural proteins or molecular systems might exhibit fractal-like self-assembly, and what functional implications could this have?
Fractal-like self-assembly may be observed in other natural proteins or molecular systems that possess repetitive structural motifs or exhibit hierarchical organization. Proteins with multiple subunits or domains that can arrange themselves in a self-similar manner across different length scales could potentially form fractal structures. For example, proteins involved in cell adhesion, extracellular matrix formation, or viral capsid assembly might exhibit fractal-like self-assembly. The functional implications of such fractal protein complexes could include increased stability, enhanced catalytic activity, or the ability to interact with multiple partners simultaneously. These fractal structures could also serve as scaffolds for organizing other biomolecules or as platforms for signal transduction processes within cells.
How might the ability to engineer fractal protein complexes be leveraged for practical applications in biotechnology or materials science?
The ability to engineer fractal protein complexes opens up exciting possibilities for applications in biotechnology and materials science. By designing proteins with specific self-assembly properties, researchers can create novel biomaterials with tailored structures and functions. Fractal protein complexes could be used as building blocks for constructing nanoscale devices, drug delivery systems, or tissue engineering scaffolds. These engineered fractal structures could also be employed in biosensors, biocatalysts, or as templates for synthesizing inorganic nanoparticles with controlled properties. Additionally, fractal protein complexes could find applications in creating new types of materials with unique mechanical, optical, or electronic properties.
Could the emergence of the citrate synthase fractal be indicative of a more general evolutionary mechanism for the generation of complex, regulatable protein structures from simpler precursors?
The emergence of the citrate synthase fractal suggests that complex, regulatable protein structures can evolve from simpler precursors through a series of genetic changes. This phenomenon may reflect a more general evolutionary mechanism where proteins undergo structural modifications that lead to the formation of intricate assemblies with new functions. The evolution of fractal protein complexes from non-fractal precursors highlights the plasticity of protein structures and the potential for proteins to acquire novel properties over time. This evolutionary process could involve gene duplication, domain shuffling, or changes in protein-protein interactions that result in the emergence of complex, self-assembling structures. The study of the citrate synthase fractal provides insights into how proteins can evolve to form sophisticated architectures that may or may not have functional significance in a biological context.
0
