Structural Insights into the Transition from Exon-Defined to Intron-Defined Spliceosome Assembly
The content discusses the structural insights into the transition from exon-defined to intron-defined spliceosome assembly. It explains that early spliceosome assembly can occur through an intron-defined pathway or an exon-defined pathway. In the exon-defined pathway, U2 binds the branch site upstream of the defined exon, and U1 snRNP interacts with the 5' splice site downstream of it. The U4/U6.U5 tri-snRNP then binds to produce a cross-exon (CE) pre-B complex, which is then converted to the spliceosomal B complex.
The content states that exon definition promotes the splicing of upstream introns and plays a key role in alternative splicing regulation. However, the three-dimensional structure of exon-defined spliceosomal complexes and the molecular mechanism of the conversion from a CE-organized to a cross-intron (CI)-organized spliceosome, a pre-requisite for splicing catalysis, remain poorly understood.
The content then describes cryo-electron microscopy analyses of human CE pre-B complex and B-like complexes, which reveal extensive structural similarities with their CI counterparts. This indicates that the CE and CI spliceosome assembly pathways converge already at the pre-B stage. Add-back experiments using purified CE pre-B complexes, coupled with cryo-electron microscopy, elucidate the order of the extensive remodelling events that accompany the formation of B complexes and B-like complexes. The molecular triggers and roles of B-specific proteins in these rearrangements are also identified.
Finally, the content shows that CE pre-B complexes can productively bind in trans to a U1 snRNP-bound 5' splice site, providing new mechanistic insights into the CE to CI switch during spliceosome assembly and its effect on pre-mRNA splice site pairing at this stage.
Customize Summary
Rewrite with AI
Generate Citations
Translate Source
To Another Language
Generate MindMap
from source content
Visit Source
www.nature.com
Structural insights into the cross-exon to cross-intron spliceosome switch - Nature
Key Insights Distilled From
by Zhen... at www.nature.com 05-22-2024
https://www.nature.com/articles/s41586-024-07458-1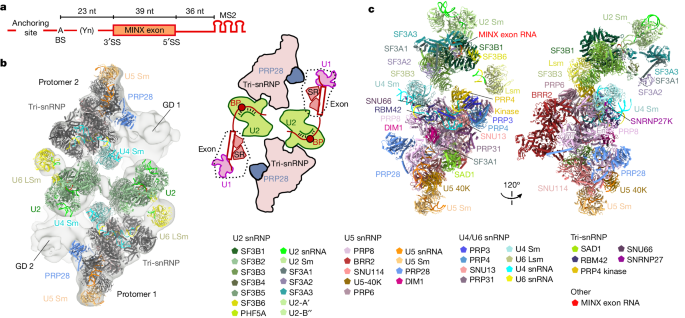
Deeper Inquiries
