Mitochondrial Complex I Activity in Microglia Driving Neuroinflammation
Core Concepts
Mitochondrial complex I activity in microglia drives neuroinflammation, highlighting its role as a potential therapeutic target for chronic inflammatory disorders of the central nervous system.
Abstract
Sustained activation of myeloid cells is a common feature in chronic neurological diseases like multiple sclerosis. Metabolic and mitochondrial features play a crucial role in guiding the activation of myeloid cells. A molecular signature identified through a multiomics approach reveals that mitochondrial complex I activity in microglia drives reverse electron transport and reactive oxygen species production, perpetuating inflammation in the central nervous system. Blocking complex I in pro-inflammatory microglia shows promise in protecting against neurotoxic damage and improving functional outcomes in animal disease models, suggesting it as a potential therapeutic target for neuroprotection in chronic inflammatory disorders.
Mitochondrial complex I activity in microglia sustains neuroinflammation - Nature
Stats
Mitochondrial complex I activity drives reverse electron transport and reactive oxygen species production.
Blocking complex I protects against neurotoxic damage and improves functional outcomes.
Quotes
Key Insights Distilled From
by L. Peruzzott... at www.nature.com 03-13-2024
https://www.nature.com/articles/s41586-024-07167-9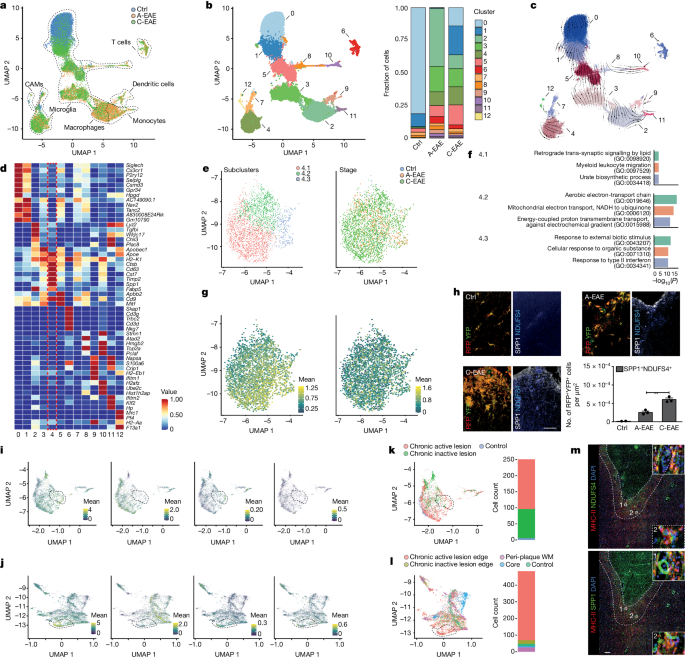
Deeper Inquiries
How can targeting mitochondrial complex I activity be translated into clinical therapies for chronic inflammatory disorders
Targeting mitochondrial complex I activity for clinical therapies in chronic inflammatory disorders involves developing specific inhibitors or modulators that can selectively block the function of this complex in microglia. By inhibiting complex I, the production of reactive oxygen species through reverse electron transport can be reduced, leading to decreased neuroinflammation and neurotoxic damage. This approach could potentially involve designing small molecules or biologics that target complex I activity specifically in pro-inflammatory microglia while sparing its essential functions in other cell types. Clinical trials would need to assess the safety, efficacy, and specificity of these compounds in treating chronic inflammatory disorders such as multiple sclerosis.
What are the potential drawbacks or limitations of blocking complex I as a therapeutic approach
While blocking mitochondrial complex I activity shows promise as a therapeutic approach for chronic inflammatory disorders, there are potential drawbacks and limitations to consider. One limitation is the possibility of off-target effects on other cellular processes that rely on complex I function for energy production and homeostasis. Complete inhibition of complex I may disrupt normal cellular metabolism and lead to unintended consequences such as impaired ATP synthesis or increased oxidative stress in non-inflammatory cells. Additionally, long-term blockade of complex I could have systemic effects beyond the central nervous system, impacting overall health and potentially causing adverse reactions. Balancing the benefits of targeting complex I with potential side effects will be crucial when considering this approach for clinical use.
How does understanding metabolic features of myeloid cells contribute to broader research on neuroinflammation
Understanding the metabolic features of myeloid cells contributes significantly to broader research on neuroinflammation by providing insights into how cellular energetics influence immune responses within the central nervous system (CNS). Metabolism plays a critical role in shaping immune cell activation states and functional outcomes during inflammation. By elucidating how metabolic pathways like mitochondrial respiration drive pro-inflammatory responses in microglia through mechanisms like reverse electron transport at complex I, researchers can identify novel targets for intervention in chronic neurological diseases characterized by sustained inflammation. This knowledge not only sheds light on disease pathogenesis but also opens up new avenues for developing precision therapies that target specific metabolic vulnerabilities unique to activated myeloid cells involved in CNS inflammation.
0
