Cefiderocol Eye Drops Shown Effective Against Deadly Pseudomonas Outbreak Strain in Experimental Models
Core Concepts
Cefiderocol, a novel "Trojan-horse" antibiotic, demonstrates potential as an effective topical treatment for corneal infections caused by the extensively drug-resistant Pseudomonas aeruginosa strain that led to a deadly outbreak in the United States.
Abstract
Researchers from the University of Pittsburgh Medical Center and Dartmouth University conducted studies evaluating the use of cefiderocol eye drops as a treatment for the multidrug-resistant Pseudomonas aeruginosa strain responsible for a recent outbreak of severe ocular infections in the US.
The outbreak, linked to contaminated artificial tear products, resulted in vision loss, surgical removal of the eye, and several deaths. The bacterial strain had not been previously reported in the country and was found to be susceptible to cefiderocol, a relatively new antibiotic approved for complicated urinary tract infections.
Through in vitro testing and an experimental rabbit model of keratitis, the researchers demonstrated that cefiderocol eye drops were non-toxic and effective against the outbreak strain. They noted that there is currently no consensus on the best antimicrobial approach for treating extensively drug-resistant keratitis, and they felt compelled to investigate cefiderocol as a potential new option.
The findings, presented at the 2024 ARVO annual meeting and published in Ophthalmology Science, suggest that topical cefiderocol could be a valuable addition to the ophthalmologist's arsenal for managing these challenging corneal infections. Further development and clinical evaluation of this antibiotic for ophthalmic use would be the next step.
Revamped Antibiotic May Treat Deadly Eye Infection
Stats
The outbreak led to loss of vision in 14 patients, surgical removal of the eyeball in four patients, and four deaths.
Cefiderocol was effective in vitro against 135 isolates from eye infections.
Cefiderocol was well tolerated on rabbit corneas and effective in the rabbit model of keratitis.
Quotes
"We showed that the 'Trojan-horse' antibiotic, cefiderocol…was non-toxic and effective against the highly resistant outbreak strain in an experimental model of infection."
"These results demonstrate that topical cefiderocol could be a new weapon in the ophthalmologist's arsenal for the treatment of corneal infections caused by highly antibiotic-resistant Pseudomonas aeruginosa."
Key Insights Distilled From
by Jake Remaly at www.medscape.com 05-07-2024
https://www.medscape.com/viewarticle/revamped-antibiotic-may-treat-deadly-eye-infection-2024a10008tx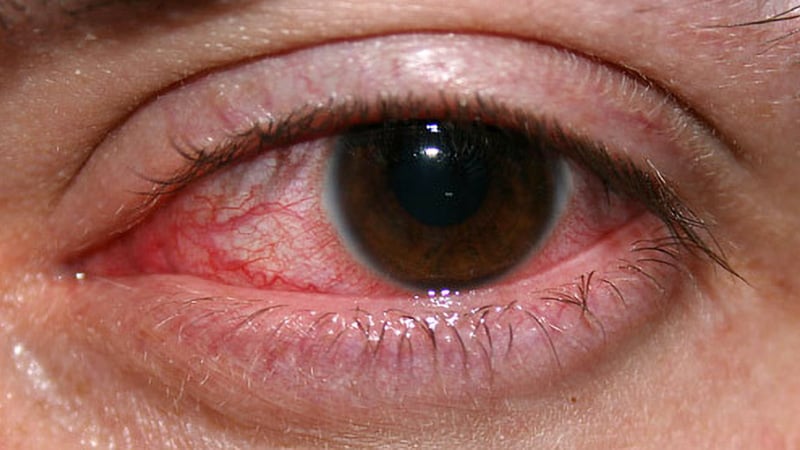
Deeper Inquiries
What are the potential mechanisms by which cefiderocol can overcome the resistance of this Pseudomonas strain?
Cefiderocol, being a "Trojan-horse" antibiotic, utilizes a unique mechanism to combat antibiotic-resistant strains like the extensively drug-resistant Pseudomonas aeruginosa. This antibiotic has a siderophore-based structure that allows it to penetrate the bacterial cell wall by mimicking iron, a vital nutrient for bacteria. Once inside the cell, cefiderocol binds to penicillin-binding proteins, inhibiting cell wall synthesis. This mechanism is distinct from traditional antibiotics, making it effective against strains that have developed resistance through mechanisms like beta-lactamase production or efflux pumps.
How do the in vitro and in vivo results with cefiderocol compare to other antibiotic options that were tried during the outbreak?
The in vitro and in vivo results of cefiderocol in combating the extensively drug-resistant Pseudomonas aeruginosa strain show promising efficacy compared to other antibiotic options used during the outbreak. In vitro testing demonstrated that cefiderocol was effective against 135 isolates from eye infections, indicating its potency against the specific strain causing the outbreak. In vivo studies using a rabbit model of keratitis further supported these findings, showing that cefiderocol was well-tolerated on rabbit corneas and effectively treated the bacterial infection. These results suggest that cefiderocol may offer a more targeted and successful treatment option compared to the mixed outcomes observed with other antibiotic regimens during the outbreak.
What are the key considerations for transitioning this experimental treatment to clinical use, and what further research is needed to support its adoption?
Transitioning the experimental treatment of cefiderocol to clinical use requires careful consideration of several key factors. Firstly, conducting rigorous clinical trials to assess the safety and efficacy of cefiderocol eye drops in human subjects is essential. These trials should evaluate the optimal dosage, frequency of administration, and potential side effects of the treatment. Additionally, regulatory approval from health authorities, such as the FDA, would be necessary to authorize the use of cefiderocol for ocular infections caused by highly antibiotic-resistant Pseudomonas aeruginosa strains.
Further research to support the adoption of cefiderocol in clinical practice should focus on conducting larger-scale clinical trials to establish its effectiveness in real-world settings. Long-term studies assessing the development of resistance, if any, and comparing cefiderocol with existing treatment options would provide valuable insights. Moreover, pharmacoeconomic studies to evaluate the cost-effectiveness of cefiderocol compared to alternative therapies would be crucial for its widespread adoption in clinical practice. Collaborative efforts between researchers, clinicians, and regulatory bodies are essential to facilitate the successful transition of cefiderocol from an experimental treatment to a clinically approved option for treating ocular infections.
0
