insight - Organic Chemistry - # Skeletal Editing of Pyrimidines to Synthesize Nitrogen-Containing Heterocycles for Drug Discovery
Innovative Skeletal Editing Method Enables Rapid Synthesis of Diverse Nitrogen-Containing Heterocyclic Compounds for Drug Discovery
Core Concepts
A new skeletal editing method can efficiently convert pyrimidine compounds into a variety of other nitrogen-containing heterocyclic ring systems, which are highly valuable structural frameworks in pharmaceutical development.
Abstract
The content discusses a novel chemical synthesis approach called "skeletal editing" that has emerged in the past decade. This innovative method enables the selective deletion, insertion, or replacement of atoms in the central framework (skeleton) of organic molecules, allowing for the alteration of their physical and chemical properties.
The article focuses on a specific skeletal editing method reported by Uhlenbruck et al. in Nature. This method can convert pyrimidine compounds, which are analogues of benzene rings with two carbon atoms replaced by nitrogen, into a variety of other nitrogen-containing "heteroarene" ring systems. These nitrogen-containing heterocycles are extremely common structural frameworks in pharmaceutical compounds, making this skeletal editing approach highly valuable for drug discovery.
The key highlights of the content are:
Skeletal editing is an attractive strategy for altering the properties of drug candidates.
Uhlenbruck et al. developed a potentially transformative skeletal editing method that can convert pyrimidines into diverse nitrogen-containing heterocyclic compounds.
Nitrogen-containing heteroarenes are extremely common in pharmaceutical compounds, so this skeletal editing method is likely to benefit drug discovery immediately.
The method enables the precise and efficient synthesis of structurally complex nitrogen-containing heterocyclic compounds, which has been limited in scope with previous approaches.
Atom-swap chemistry speeds synthesis of compounds for drug discovery
Stats
Nitrogen-containing heteroarenes are extremely common structural frameworks in pharmaceutical compounds.
The authors' skeletal editing method can convert pyrimidines, which are analogues of benzene rings with two carbon atoms replaced by nitrogen, into a variety of other nitrogen-containing heterocyclic ring systems.
Quotes
"In the past decade, a new concept in chemical synthesis has emerged, in which the ordinarily unreactive central framework — the skeleton — of organic molecules is altered through the selective deletion, insertion or replacement of atoms1."
"Given that nitrogen-containing heteroarenes are extremely common structural frameworks in pharmaceutical compounds, the authors' method is likely to benefit drug discovery immediately."
Key Insights Distilled From
by Ánge... at www.nature.com 07-03-2024
https://www.nature.com/articles/d41586-024-02017-0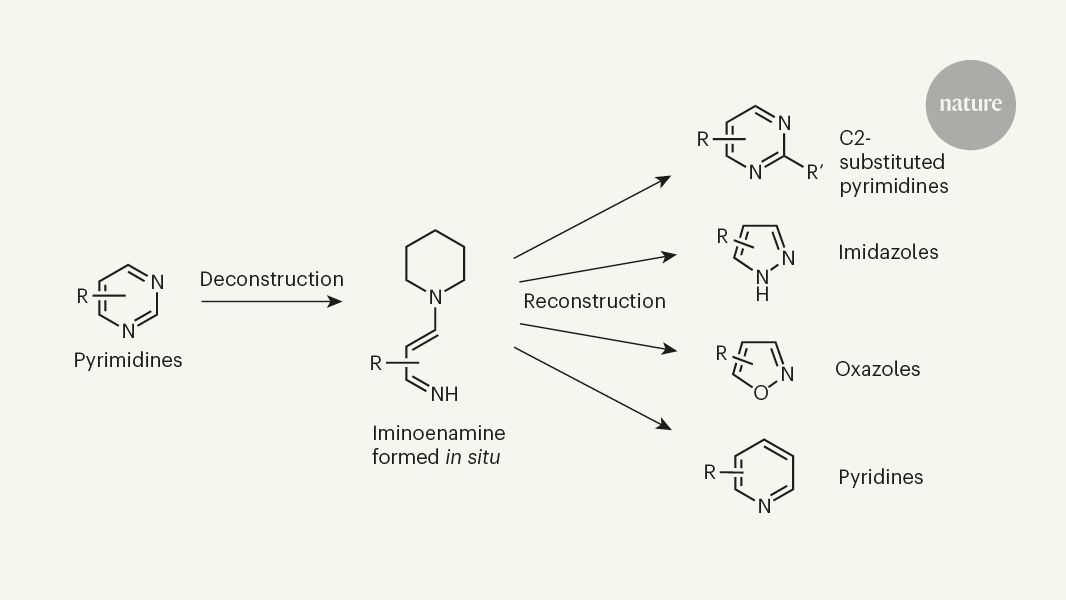
Deeper Inquiries
How can this skeletal editing method be further expanded to enable the synthesis of an even broader range of heterocyclic compounds for drug discovery?
The skeletal editing method described in the context can be expanded by exploring different substitution patterns and functional groups that can be incorporated into the heterocyclic compounds. By understanding the reactivity of different atoms and groups within the molecules, researchers can design specific transformations that allow for the creation of diverse heterocyclic structures. Additionally, the development of new catalysts or reaction conditions that facilitate the selective modification of specific bonds can broaden the scope of this method. Furthermore, exploring the use of different starting materials and building blocks can also lead to the synthesis of a wider range of heterocyclic compounds with potential pharmaceutical applications.
What are the potential limitations or challenges in applying this skeletal editing approach to structurally complex drug candidates?
One potential limitation of applying the skeletal editing approach to structurally complex drug candidates is the selectivity of the reactions. When dealing with intricate molecules, there is a higher chance of unwanted side reactions or unintended modifications occurring. Ensuring the precise control of the skeletal editing process in complex structures can be challenging and may require the development of highly selective catalysts or reaction conditions. Another challenge is scalability, as the method may not be easily adaptable to large-scale synthesis of complex drug candidates. Additionally, the compatibility of the skeletal editing approach with other functional groups present in the molecule needs to be carefully considered to avoid undesired interactions or changes to the overall pharmacological properties of the compound.
How might this skeletal editing technology inspire the development of new computational tools or algorithms to assist in the rational design of novel drug-like molecules?
The advancement of skeletal editing technology could inspire the development of computational tools or algorithms that predict the outcomes of specific skeletal modifications in molecules. By analyzing the reactivity patterns of different atoms and bonds, computational models can be created to guide researchers in designing novel drug-like molecules with desired properties. Machine learning algorithms could be trained on a database of known reactions and their outcomes to predict the feasibility and selectivity of skeletal editing transformations. Additionally, molecular modeling software could be enhanced to simulate the effects of skeletal modifications on the three-dimensional structure and interactions of the molecules, aiding in the rational design of new drug candidates. This integration of experimental data with computational tools could streamline the drug discovery process and lead to the more efficient development of novel pharmaceutical compounds.
0
