Counterfeit Ozempic Discovered in Europe and the USA, Posing Risks to Patients
Core Concepts
Counterfeit versions of the diabetes and obesity medication Ozempic have been discovered in the official supply chains in Germany, Brazil, the UK, and the USA, posing potential health risks to patients.
Abstract
The World Health Organization (WHO) has reported the discovery of several counterfeit batches of the diabetes and obesity medication Ozempic in late 2023. These counterfeit products were found in the official supply chains in Germany, Brazil, the United Kingdom, and the United States.
The manufacturer, Novo Nordisk, has confirmed that these batches are not original Ozempic products. The counterfeit pens may be ineffective or contain harmful ingredients, though the full toxicology results are currently unknown.
The high demand for Ozempic has exceeded the production capacity, leading to supply shortages. In response, Novo Nordisk will temporarily restrict the availability of the lower-dose Ozempic 0.25 mg and 0.5 mg formulations in the second quarter of 2024, focusing on providing the 1 mg dose for those already being treated.
The WHO has provided guidance to healthcare professionals on identifying counterfeit Ozempic products, including checking for suspicious batch numbers, protruding scales on the pens, poor label adhesion, and spelling errors. Pharmacists are advised to open the packaging and verify the authenticity of the product before dispensing.
In Germany, the Federal Institute for Drugs and Medical Devices (BfArM) has also implemented the securPharm system, which uses unique serial numbers, product codes, and other identifiers to verify the authenticity of drug packages throughout the supply chain.
WHO Warns Against Counterfeit Ozempic in Europe and the USA
Stats
The batch number LP6F832 does not exist.
The combination of batch number NAR0074 with serial number 430834149057 does not match manufacturing records.
The batch number MP5E511 is correct, but the product is counterfeit.
Quotes
"The sustained high demand for Ozempic exceeds production capacities," wrote Novo Nordisc.
"To ensure the supply of Ozempic 1 mg, particularly for individuals already being treated with Ozempic for type 2 diabetes, the focus is on providing Ozempic 1 mg in agreement with the relevant authorities."
Key Insights Distilled From
by Michael at www.medscape.com 07-03-2024
https://www.medscape.com/viewarticle/who-warns-against-counterfeit-ozempic-europe-and-united-2024a1000caq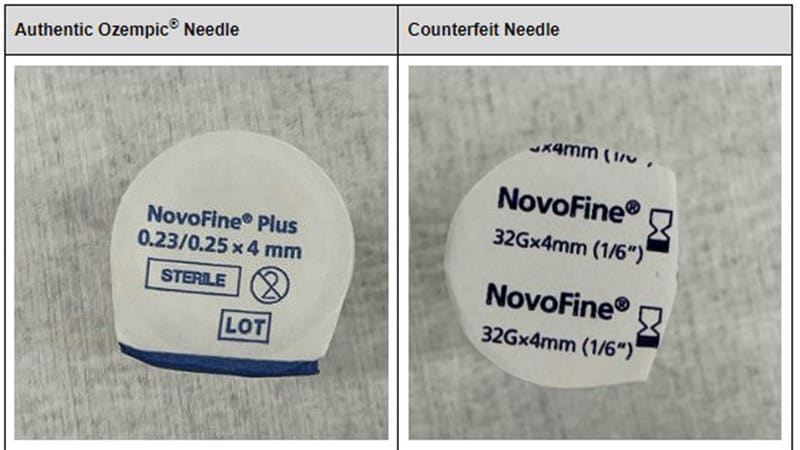
Deeper Inquiries
What measures can be taken to improve the security and traceability of pharmaceutical supply chains to prevent the proliferation of counterfeit medications?
To enhance the security and traceability of pharmaceutical supply chains and mitigate the spread of counterfeit medications, several measures can be implemented:
Serialization and Track-and-Trace Technologies: Implementing serialization, which involves assigning a unique identifier to each drug package, and track-and-trace technologies can help monitor the movement of pharmaceutical products throughout the supply chain. This enables stakeholders to verify the authenticity of medications and track their journey from manufacturing to distribution.
Tamper-Evident Packaging: Utilizing tamper-evident seals and packaging can help ensure that medications have not been altered or tampered with during transit. Any signs of tampering can alert healthcare providers and patients to potential counterfeit products.
Blockchain Technology: Leveraging blockchain technology can create an immutable and transparent record of transactions within the supply chain. By recording each step in the drug distribution process on a blockchain, stakeholders can verify the authenticity of medications and identify any discrepancies or unauthorized changes.
Collaboration and Information Sharing: Establishing partnerships between pharmaceutical companies, regulatory authorities, healthcare providers, and law enforcement agencies can facilitate the exchange of information on counterfeit trends, enabling swift action to be taken against illicit drug activities.
Education and Training: Providing comprehensive training to pharmacists, healthcare providers, and patients on how to identify counterfeit medications and verify the authenticity of drugs can empower them to detect and report suspicious products effectively.
Regulatory Oversight: Strengthening regulatory oversight and enforcement measures can deter counterfeiters and ensure compliance with stringent quality standards and security protocols in the pharmaceutical supply chain.
How can healthcare providers and patients be better educated on the risks of counterfeit drugs and the importance of verifying the authenticity of their medications?
To enhance awareness among healthcare providers and patients regarding the risks associated with counterfeit drugs and the significance of verifying medication authenticity, the following strategies can be employed:
Training Programs: Develop educational programs and workshops for healthcare providers to educate them on the prevalence of counterfeit medications, the potential risks to patient health, and methods to identify suspicious products.
Patient Awareness Campaigns: Launch targeted awareness campaigns aimed at patients to educate them on the dangers of counterfeit drugs, the importance of obtaining medications from reputable sources, and how to verify the authenticity of their prescriptions.
Informational Materials: Distribute brochures, posters, and online resources that outline the characteristics of counterfeit medications, provide guidance on verifying drug authenticity, and encourage reporting of suspicious products.
Pharmacist Guidance: Encourage pharmacists to engage with patients during medication dispensing, emphasizing the need to scrutinize packaging, labels, and product features to ensure they are receiving genuine medications.
Digital Tools: Utilize digital platforms, such as mobile apps or websites, to disseminate information on counterfeit drugs, offer resources for authentication, and raise awareness about the risks associated with counterfeit pharmaceuticals.
Collaboration with Patient Advocacy Groups: Partner with patient advocacy organizations to amplify messaging on counterfeit drug risks, leverage their networks to reach a broader audience, and foster a community-driven approach to combatting counterfeit medications.
What role can emerging technologies, such as blockchain or serialization, play in enhancing the integrity of the pharmaceutical supply chain and protecting patients from counterfeit products?
Emerging technologies like blockchain and serialization offer significant potential in bolstering the integrity of the pharmaceutical supply chain and safeguarding patients from counterfeit products:
Blockchain for Transparency: Blockchain technology can create a decentralized and transparent ledger that records every transaction within the supply chain. This immutable record enhances transparency, enabling stakeholders to trace the origin of medications, verify authenticity, and detect any unauthorized alterations.
Serialization for Unique Identification: Serialization involves assigning a unique identifier to each drug package, allowing for individual tracking and verification. By implementing serialization, pharmaceutical companies can ensure that each product is authentic and has not been tampered with during transit.
Data Security and Privacy: Blockchain's secure and encrypted nature protects sensitive information, such as patient data and drug manufacturing details, from unauthorized access or tampering. This enhances data security and privacy within the supply chain, reducing the risk of counterfeit infiltration.
Real-Time Monitoring: Serialization and blockchain technologies enable real-time monitoring of drug movements, allowing for immediate detection of counterfeit products or unauthorized diversions. This proactive approach enhances supply chain visibility and responsiveness to potential threats.
Authentication and Verification: By leveraging blockchain and serialization, patients, healthcare providers, and regulators can authenticate medications, verify their legitimacy, and ensure adherence to quality standards. This empowers stakeholders to make informed decisions and mitigate the risks associated with counterfeit drugs.
Compliance and Regulatory Alignment: Implementing blockchain and serialization aligns with regulatory requirements, such as the EU Falsified Medicines Directive, enhancing compliance and ensuring that pharmaceutical supply chains adhere to stringent quality and security standards to protect patient safety.
0
