approfondimento - Computational Biology - # Structural and Functional Analysis of the Lysosomal Cholesterol Sensor Protein LYCHOS
Structural Insights into the Hybrid Transmembrane Protein LYCHOS, a Cholesterol Sensor Regulating Cellular Metabolism and Growth
Concetti Chiave
LYCHOS is a unique hybrid transmembrane protein that combines a plant-like auxin transporter domain and a G-protein-coupled receptor (GPCR) domain to sense cholesterol and regulate the master metabolic regulator mTORC1.
Sintesi
The article presents a detailed structural and functional analysis of the human protein LYCHOS (GPR155), which is a lysosomal transmembrane protein that acts as a cholesterol sensor. Key insights from the study:
LYCHOS has a unique hybrid structure, comprising a transporter-like domain fused to a GPCR domain. This is an unusual example of a GPCR being part of a larger transmembrane assembly.
The transporter-like domain of LYCHOS is an orthologue of the plant PIN-FORMED (PIN) auxin transporter family, showing greater structural similarity to plant auxin transporters than to known human transporters.
Cholesterol sensing by LYCHOS is mediated by a conserved cholesterol-binding motif positioned between the GPCR and transporter domains.
The coordinated functions of the LYCHOS transporter and GPCR domains are proposed to enable cholesterol sensing and regulation of the master metabolic regulator mTORC1.
The study provides important structural insights into the unique hybrid architecture of LYCHOS and its role as a cholesterol sensor in regulating eukaryotic metabolism and cell growth.
LYCHOS is a human hybrid of a plant-like PIN transporter and a GPCR - Nature
Statistiche
LYCHOS functions as a cholesterol sensor to facilitate the cholesterol-dependent activation of the master protein kinase mTORC1.
LYCHOS has a homodimeric transmembrane assembly with a transporter-like domain fused to a G-protein-coupled receptor (GPCR) domain.
The LYCHOS transporter-like domain is an orthologue of the plant PIN-FORMED (PIN) auxin transporter family.
Citazioni
"LYCHOS (GPR155) is a lysosomal transmembrane protein that functions as a cholesterol sensor, facilitating the cholesterol-dependent activation of the master protein kinase mechanistic target of rapamycin complex 1 (mTORC1)."
"We reveal that the LYCHOS transporter-like domain is an orthologue of the plant PIN-FORMED (PIN) auxin transporter family, and has greater structural similarity to plant auxin transporters than to known human transporters."
Approfondimenti chiave tratti da
by Charles Bayl... alle www.nature.com 10-02-2024
https://www.nature.com/articles/s41586-024-08012-9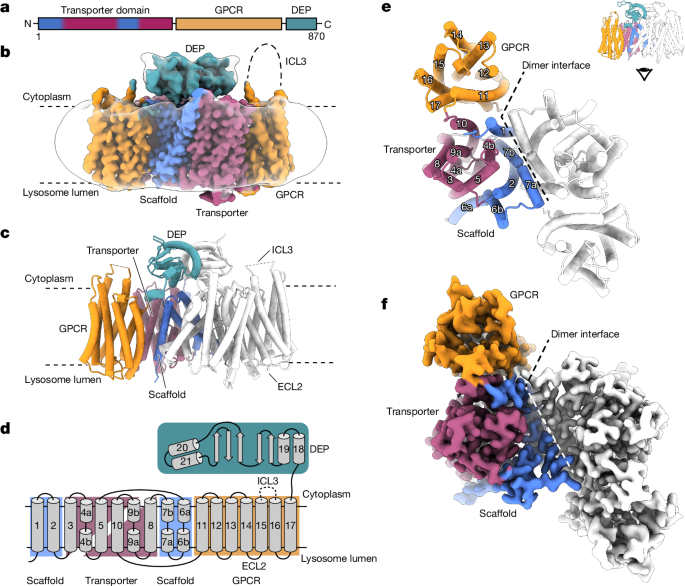
Domande più approfondite
How do the structural and functional features of the LYCHOS transporter and GPCR domains enable the integration of cholesterol sensing and mTORC1 regulation?
The structural and functional features of the LYCHOS transporter and GPCR domains are intricately designed to facilitate the integration of cholesterol sensing and mTORC1 regulation. The LYCHOS protein exhibits a homodimeric transmembrane assembly, where the transporter-like domain is fused to a class B2-like GPCR domain. This unique configuration allows for a direct interaction between the two domains, enabling them to work in concert.
The transporter-like domain, which is an orthologue of the plant PIN-FORMED (PIN) auxin transporter family, possesses a conserved cholesterol-binding motif that is strategically positioned between the GPCR and transporter domains. This motif is crucial for cholesterol sensing, as it allows LYCHOS to detect changes in cholesterol levels within the lysosomal membrane. When cholesterol binds to this motif, it induces conformational changes that activate the GPCR domain.
The activation of the GPCR domain subsequently leads to the recruitment and activation of the mechanistic target of rapamycin complex 1 (mTORC1), a master regulator of cell growth and metabolism. This coordinated mechanism ensures that the cell can effectively respond to fluctuations in cholesterol availability, thereby linking nutrient sensing to metabolic regulation. The structural integration of these domains exemplifies a sophisticated evolutionary adaptation that enhances cellular responsiveness to environmental changes.
What are the potential implications of the plant-like structural features of the LYCHOS transporter domain for understanding the evolutionary origins of cholesterol sensing mechanisms in eukaryotes?
The plant-like structural features of the LYCHOS transporter domain provide significant insights into the evolutionary origins of cholesterol sensing mechanisms in eukaryotes. The resemblance of the LYCHOS transporter to the plant PIN auxin transporters suggests that there may be a conserved evolutionary pathway that links plant and animal systems in their ability to sense and respond to lipid signals.
This structural similarity implies that the mechanisms for lipid sensing, including cholesterol, may have evolved from a common ancestral transporter system that was adapted for different functions in plants and animals. The presence of a cholesterol-binding motif in a transporter domain that shares characteristics with plant proteins indicates that eukaryotic cells may have repurposed existing transport mechanisms to develop specialized functions related to cholesterol metabolism and signaling.
Understanding these evolutionary connections could lead to new perspectives on how lipid sensing evolved in multicellular organisms and may reveal fundamental principles governing cellular communication and metabolic regulation across different life forms. This knowledge could also inform research into the development of therapeutic strategies targeting cholesterol-related diseases by leveraging insights from both plant and animal biology.
Could the unique hybrid architecture of LYCHOS inspire the design of novel synthetic biology or biotechnology applications involving the fusion of diverse functional domains?
The unique hybrid architecture of LYCHOS presents exciting opportunities for the design of novel synthetic biology and biotechnology applications. The integration of a transporter-like domain with a GPCR domain exemplifies how diverse functional elements can be combined to create sophisticated signaling systems. This concept can be applied to synthetic biology, where researchers can engineer new proteins that mimic the functionality of LYCHOS to achieve specific cellular responses.
For instance, by fusing different sensing domains with effector domains, scientists could create synthetic receptors that respond to a variety of environmental signals, such as nutrients, toxins, or hormones. This could lead to the development of biosensors capable of detecting specific metabolites or signaling molecules in real-time, which would be invaluable in fields such as environmental monitoring, agriculture, and medical diagnostics.
Moreover, the principles derived from the LYCHOS architecture could inspire the creation of synthetic pathways that regulate metabolic processes in engineered organisms. By designing hybrid proteins that integrate sensing and signaling functions, researchers could manipulate cellular behavior to optimize production processes in biotechnology, such as the synthesis of biofuels or pharmaceuticals.
In summary, the innovative design of LYCHOS not only enhances our understanding of cholesterol sensing and mTORC1 regulation but also serves as a blueprint for future advancements in synthetic biology, potentially leading to groundbreaking applications that harness the power of engineered biological systems.
0
