Using Patient-Derived Brain Organoids to Identify Potential Treatments for the Genetic Disorder Timothy Syndrome
The article discusses the use of brain organoids, which are complex 3D neural tissues generated from patient-derived induced pluripotent stem cells, as a model system to understand disease mechanisms and test potential therapies for neurological conditions.
The authors focus on Timothy syndrome, a severe developmental disorder with limited treatment options. By generating brain organoids from patients with Timothy syndrome, the researchers were able to gain insights into the underlying disease mechanisms. They found that the genetic mutation responsible for Timothy syndrome leads to the aberrant splicing of an ion channel gene, resulting in disrupted neuronal function.
Importantly, the researchers were able to use the brain organoid model to identify a potential therapeutic strategy - targeting the aberrant RNA splicing with antisense oligonucleotides. This approach was able to rescue the neuronal defects observed in the Timothy syndrome brain organoids, suggesting it could be a promising avenue for developing treatments for this condition.
The use of patient-derived brain organoids represents a powerful tool for understanding complex neurological disorders and accelerating the development of effective therapies. This study demonstrates the value of this approach in identifying targeted RNA-based interventions for genetic disorders that lack adequate treatment options.
要約をカスタマイズ
AI でリライト
引用を生成
原文を翻訳
他の言語に翻訳
マインドマップを作成
原文コンテンツから
原文を表示
www.nature.com
Targeting RNA opens therapeutic avenues for Timothy syndrome
抽出されたキーインサイト
by Silvia Velas... 場所 www.nature.com 04-24-2024
https://www.nature.com/articles/d41586-024-00911-1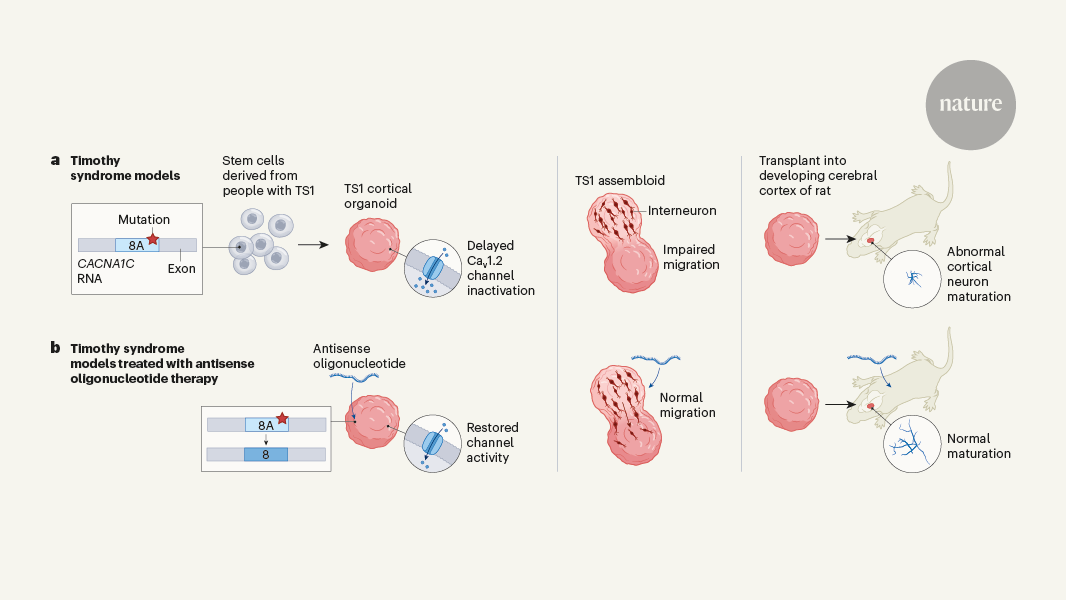
深掘り質問
