FDA Grants Breakthrough Designation to Roche's Elecsys Neurofilament Light Chain Test for MS
核心概念
Early detection of MS activity is crucial for disease management and patient outcomes.
要約
The FDA has granted breakthrough device designation to Roche's Elecsys Neurofilament Light Chain (NfL) test for multiple sclerosis (MS). The test aids in detecting disease activity in adults with relapsing-remitting or secondary progressive MS. Rising NfL levels indicate neuroaxonal injury and correlate with MS disease activity. The test offers insights for disease management and treatment monitoring. Elevated NfL levels at baseline are associated with increased future disability risk. The Elecsys NfL test provides a minimally invasive blood draw with rapid results, potentially improving outcomes for MS patients.
要約をカスタマイズ
AI でリライト
引用を生成
原文を翻訳
他の言語に翻訳
マインドマップを作成
原文コンテンツから
原文を表示
www.medscape.com
Blood Test for MS Activity Gets FDA Breakthrough Designation
統計
"The test is intended to be used as an aid in detection of disease activity in adults aged 18-55 years with relapsing-remitting multiple sclerosis (RRMS) or secondary progressive multiple sclerosis (SPMS)."
"Rising NfL levels are a known indicator of neuroaxonal injury and correlate with MS disease activity."
"An analysis of NfL in two large MS cohorts found that elevated levels of the neuronal protein at baseline were associated with large increases in future disability risk."
引用
"For patients with RRMS and SPMS, detection of disease activity is critically important in enabling them and their physicians to make the best possible decisions for the management of the disease." - Roche
"The Elecsys NfL test could help laboratories to scale MS testing on widely available, fully automated, and standardized Roche cobas instruments with the confidence of in-vitro diagnostics quality in a timely manner." - Roche
抽出されたキーインサイト
by Megan Brooks 場所 www.medscape.com 11-15-2023
https://www.medscape.com/viewarticle/998500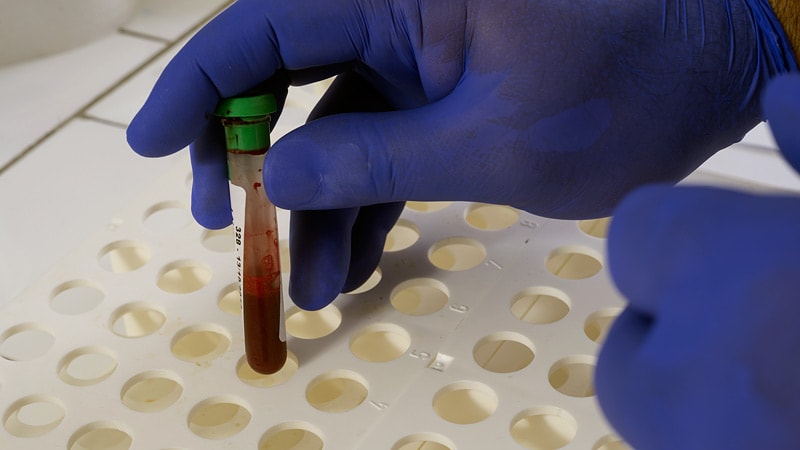
深掘り質問
How can early detection of MS activity impact long-term patient outcomes?
Early detection of MS activity can significantly impact long-term patient outcomes by allowing for timely intervention and personalized treatment plans. Monitoring NfL levels through blood tests can provide insights into disease progression, response to therapies, and overall disease management. By detecting changes in NfL levels early, healthcare providers can adjust treatment strategies promptly, potentially slowing down disease progression, reducing relapse rates, and improving quality of life for patients with MS. Additionally, early detection can help in identifying patients at higher risk of disability progression, enabling proactive measures to be taken to mitigate long-term consequences of the disease.
What potential challenges or limitations could arise from relying solely on NfL levels for disease management decisions?
While NfL levels can serve as a valuable biomarker for MS activity, relying solely on these levels for disease management decisions may present certain challenges and limitations. One limitation is the need for further research to establish standardized reference ranges and thresholds for interpreting NfL levels accurately in different patient populations. Additionally, NfL levels may not always reflect the full spectrum of disease activity or treatment response in MS patients, as other factors such as inflammation, lesion burden, and clinical symptoms also play a role in disease progression. Therefore, using NfL levels as a complementary tool alongside clinical assessments and imaging studies is crucial to ensure comprehensive disease management and treatment decision-making.
How might advancements in blood testing technology for MS contribute to broader healthcare innovations?
Advancements in blood testing technology for MS, such as the Elecsys NfL test, have the potential to revolutionize disease management not only for MS but also for other neurological conditions and beyond. By offering a minimally invasive and rapid method for monitoring disease activity, these innovations can improve patient outcomes, enhance treatment efficacy, and optimize healthcare resource utilization. Furthermore, the scalability of blood testing technology on automated platforms like Roche cobas instruments can streamline diagnostic processes, increase testing accessibility, and facilitate data-driven decision-making in clinical practice. These advancements pave the way for personalized medicine approaches, precision healthcare interventions, and the development of novel therapeutics based on biomarker insights, ultimately driving healthcare innovations across various medical specialties.
0
