Atomic-Resolution Imaging Reveals Intricate Surface Structure and Premelting Dynamics of Hexagonal Ice
핵심 개념
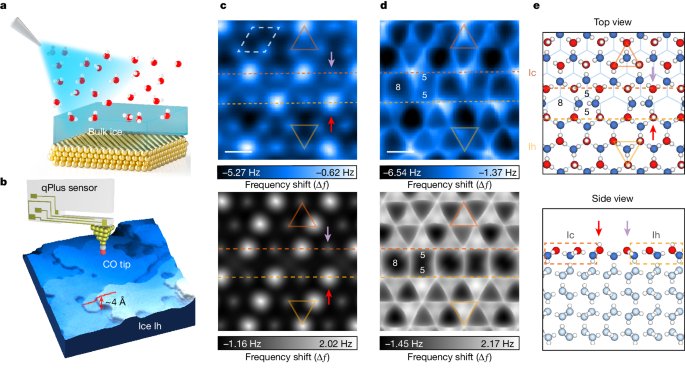
The ice surface consists of a complex mixture of hexagonal (Ih) and cubic (Ic) stacking nanodomains, which undergo gradual disordering and premelting at temperatures above 120 Kelvin, originating from defective boundaries between the Ih and Ic regions.
초록
The article presents groundbreaking findings from atomic-resolution imaging of the basal (0001) surface of hexagonal water ice (ice Ih) using cryogenic atomic force microscopy with a carbon monoxide-functionalized tip. The key insights are:
-
The ice-Ih surface exhibits a complex nanostructure, comprising a mixture of Ih- and cubic Ic-stacking domains, forming a periodic (\sqrt{19}\times \sqrt{19}) superstructure.
-
Density functional theory analysis reveals that this reconstructed surface is stabilized by minimizing the electrostatic repulsion between dangling OH bonds, compared to the ideal ice surface.
-
As temperature increases above 120 Kelvin, the ice surface gradually becomes disordered, indicating the onset of the premelting process.
-
The premelting originates from the defective boundaries between the Ih and Ic domains and can be promoted by the formation of a planar local structure.
These results provide unprecedented atomic-scale insights into the long-standing mystery of ice surface structures and the molecular mechanisms underlying the premelting phenomenon. This knowledge can lead to a paradigm shift in understanding ice physics and chemistry, with implications for diverse fields such as atmospheric science, cryobiology, and tribology.
Imaging surface structure and premelting of ice Ih with atomic resolution - Nature
통계
The ice surface consists of a mixture of hexagonal (Ih) and cubic (Ic) stacking nanodomains.
The reconstructed ice surface forms a (\sqrt{19}\times \sqrt{19}) periodic superstructure.
The premelting process starts at temperatures above 120 Kelvin.
인용구
"We find that the crystalline ice-Ih surface consists of mixed Ih- and cubic (Ic)-stacking nanodomains, forming (\sqrt{19}\times \sqrt{19}) periodic superstructures."
"Density functional theory reveals that this reconstructed surface is stabilized over the ideal ice surface mainly by minimizing the electrostatic repulsion between dangling OH bonds."
"Moreover, we observe that the ice surface gradually becomes disordered with increasing temperature (above 120 Kelvin), indicating the onset of the premelting process."
더 깊은 질문
How do the structural and dynamic properties of the ice surface influence its interactions with other molecules, such as gases, aerosols, or biological entities?
The structural and dynamic properties of the ice surface play a crucial role in determining its interactions with other molecules. The atomic-resolution imaging of the ice Ih surface revealed a mixed stacking of Ih and Ic nanodomains, forming periodic superstructures. These surface structures create specific binding sites and surface energies that can influence the adsorption and desorption of molecules. For example, the presence of dangling OH bonds and the reconstructed surface can affect the adsorption of gases by providing sites for interaction through hydrogen bonding or van der Waals forces. Additionally, the observed premelting process at the ice surface can lead to the formation of liquid-like layers, altering the surface properties and enhancing the adsorption of molecules. Understanding these structural and dynamic properties is essential for predicting the behavior of ice surfaces in various environments and their interactions with different molecules.
What are the potential implications of the discovered ice surface reconstruction and premelting mechanisms for the understanding of atmospheric and cryospheric processes?
The discovery of the ice surface reconstruction and premelting mechanisms has significant implications for the understanding of atmospheric and cryospheric processes. The reconstructed surface structure of ice Ih, stabilized by minimizing electrostatic repulsion between dangling OH bonds, provides insights into the surface properties that can influence interactions with atmospheric gases and aerosols. The observed premelting process, initiated at defective boundaries between Ih and Ic domains, can impact the formation of liquid-like layers on ice surfaces, affecting processes such as cloud formation and atmospheric reactions. Understanding these mechanisms can improve models of ice-atmosphere interactions, leading to more accurate predictions of climate dynamics, cloud formation, and chemical reactions in the atmosphere. Moreover, insights into ice premelting can enhance our understanding of cryospheric processes, such as ice melting and glacier dynamics, contributing to better assessments of global climate change impacts.
Could the insights gained from this study be leveraged to develop novel materials or surfaces with tailored wetting, adhesion, or friction properties?
The insights gained from the study on ice surface reconstruction and premelting mechanisms hold great potential for the development of novel materials or surfaces with tailored wetting, adhesion, or friction properties. By understanding the atomic-scale structures and dynamics of ice surfaces, researchers can apply similar principles to engineer surfaces with specific functionalities. For example, the knowledge of how surface reconstruction stabilizes the ice Ih surface could inspire the design of materials with controlled surface structures to enhance wetting properties or promote specific molecular interactions. The observed premelting process and its influence on surface disorder could be leveraged to develop surfaces with tunable adhesion properties or reduced friction for various applications. By mimicking the principles governing ice surface interactions, novel materials could be designed for applications in anti-icing coatings, lubricants, or biomimetic surfaces with tailored properties. Overall, the study's findings open up possibilities for the development of advanced materials based on the fundamental understanding of ice surface properties.