통찰 - Neuroscience - # Astrocyte Calcium Signaling and Network Dynamics in Response to Neurotransmitters
Astrocyte Networks Encode Local Neurotransmitter Signals Across Cortical Regions and Time Scales
핵심 개념
Local, transient neurotransmitter inputs are encoded by broad cortical astrocyte networks over a minutes-long time course, contributing to astrocyte-neuron communication across slow, network-level spatiotemporal scales.
초록
The article investigates how astrocytes, the most abundant non-neuronal cells in the mammalian brain, respond to and modulate neuronal activity through calcium (Ca2+) signaling. Astrocyte Ca2+ activity occurs across multiple spatiotemporal scales, from fast, subcellular activity to slow, synchronized activity across connected astrocyte networks. However, the inputs that drive astrocyte network dynamics remain unclear.
The researchers used ex vivo and in vivo two-photon astrocyte imaging to study how astrocytes respond to neuronal neurotransmitter inputs at multiple spatiotemporal scales. They found that brief, subcellular inputs of GABA and glutamate lead to widespread, long-lasting astrocyte Ca2+ responses beyond the individual stimulated cell. Further, they discovered that a key subset of Ca2+ activity, known as propagative activity, differentiates astrocyte network responses to these two main neurotransmitters, and may influence responses to future inputs.
The results demonstrate that local, transient neurotransmitter inputs are encoded by broad cortical astrocyte networks over a minutes-long time course, contributing to the growing evidence that substantial astrocyte-neuron communication occurs across slow, network-level spatiotemporal scales. These findings will enable future studies to investigate the link between specific astrocyte Ca2+ activity and specific functional outputs, which could build a consistent framework for astrocytic modulation of neuronal activity.
Network-level encoding of local neurotransmitters in cortical astrocytes - Nature
통계
Astrocytes are the most abundant non-neuronal cell type in the mammalian brain.
Astrocyte Ca2+ activity occurs across multiple spatiotemporal scales, from fast, subcellular activity to slow, synchronized activity across connected astrocyte networks.
Brief, subcellular inputs of GABA and glutamate lead to widespread, long-lasting astrocyte Ca2+ responses beyond the individual stimulated cell.
A key subset of Ca2+ activity, known as propagative activity, differentiates astrocyte network responses to GABA and glutamate.
인용구
"Astrocytes, the most abundant non-neuronal cell type in the mammalian brain, are crucial circuit components that respond to and modulate neuronal activity through calcium (Ca2+) signalling1,2,3,4,5,6,7."
"Together, our results demonstrate that local, transient neurotransmitter inputs are encoded by broad cortical astrocyte networks over a minutes-long time course, contributing to accumulating evidence that substantial astrocyte–neuron communication occurs across slow, network-level spatiotemporal scales12,13,14."
핵심 통찰 요약
by Michelle K. ... 게시일 www.nature.com 04-17-2024
https://www.nature.com/articles/s41586-024-07311-5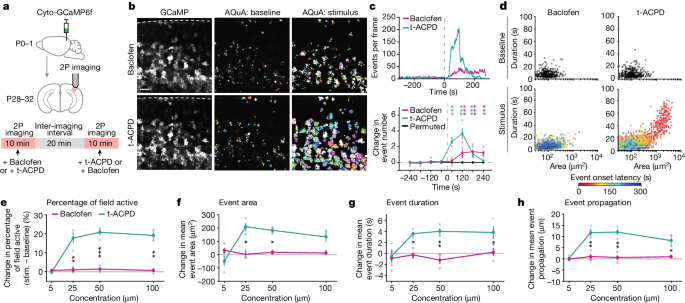
더 깊은 질문
How do the specific patterns of astrocyte Ca2+ activity, such as propagative activity, influence the functional outputs of astrocyte-neuron communication?
The specific patterns of astrocyte Ca2+ activity, including propagative activity, play a crucial role in influencing the functional outputs of astrocyte-neuron communication. Propagative activity, which differentiates astrocyte network responses to neurotransmitters like GABA and glutamate, can lead to widespread and long-lasting astrocyte Ca2+ responses beyond the initially stimulated cell. This phenomenon suggests that astrocytes can integrate and propagate signals across networks, affecting multiple neighboring cells. By doing so, astrocytes can modulate neuronal activity in a coordinated manner, impacting processes such as synaptic transmission, plasticity, and overall circuit function. Therefore, understanding how these specific patterns of Ca2+ activity influence astrocyte-neuron communication is essential for unraveling the complexities of brain function.
What are the potential implications of the network-level encoding of neurotransmitter signals by astrocytes for our understanding of brain function and dysfunction?
The network-level encoding of neurotransmitter signals by astrocytes has significant implications for our understanding of brain function and dysfunction. By demonstrating that local, transient neurotransmitter inputs are encoded by broad cortical astrocyte networks over a minutes-long time course, this study highlights the intricate role of astrocytes in information processing within the brain. This finding suggests that astrocytes are not merely supportive cells but active participants in regulating neuronal activity and circuit dynamics. Understanding how astrocytes encode and transmit neurotransmitter signals at a network level can provide insights into various brain functions, such as sensory processing, learning, and memory. Moreover, dysregulation of astrocyte-mediated neurotransmitter encoding could contribute to neurological disorders, emphasizing the importance of studying astrocyte function in both health and disease states.
Could the insights from this study on astrocyte network dynamics be applied to other types of glial cells or non-neuronal brain cells to gain a more comprehensive understanding of brain information processing?
The insights gained from studying astrocyte network dynamics could potentially be applied to other types of glial cells or non-neuronal brain cells to enhance our understanding of brain information processing. Glial cells, including astrocytes, microglia, and oligodendrocytes, play diverse roles in supporting neuronal function and maintaining brain homeostasis. Given the intricate interactions between different glial cell types and neurons, understanding how astrocytes encode neurotransmitter signals at a network level may provide a framework for investigating similar processes in other glial cells. By studying the network dynamics of various glial cell populations, researchers can uncover how different glial cells contribute to information processing, synaptic plasticity, and overall brain function. This comparative approach could lead to a more comprehensive understanding of the complex cellular interactions that underlie brain activity and may offer new insights into neurological disorders where glial cell dysfunction is implicated.
0
