통찰 - Pharmaceutical Research - # Agonist Antibody Targeting NPR1 Receptor for Heart Failure Treatment
Investigational Monoclonal Agonist Antibody REGN5381 Targets NPR1 Receptor to Regulate Vascular Tone and Treat Heart Failure
핵심 개념
An investigational monoclonal agonist antibody REGN5381 targets the membrane-bound guanylate cyclase receptor NPR1 to induce active-like receptor conformation, resulting in hemodynamic effects that preferentially lower venous pressures to treat heart failure.
초록
The content discusses the development of an investigational monoclonal agonist antibody called REGN5381 that targets the NPR1 receptor. NPR1 is the receptor for natriuretic peptides, which are counter-regulatory hormones released in heart failure to induce vasodilation, diuresis, and natriuresis, thereby lowering venous pressures and relieving venous congestion.
The key highlights are:
- Genetic analysis of over 700,000 individuals showed that lifelong exposure to coding variants of the NPR1 gene is associated with changes in blood pressure and risk of heart failure.
- REGN5381 is an allosteric agonist of NPR1 that induces an active-like receptor conformation, leading to hemodynamic effects that preferentially target the venous vasculature.
- In animal models, REGN5381 reduced systolic blood pressure and venous pressure without obvious changes in diuresis and natriuresis.
- In healthy human volunteers, REGN5381 produced the expected hemodynamic effects reflecting reductions in venous pressures, without obvious changes in diuresis and natriuresis.
- These data support the development of REGN5381 for long-lasting and selective lowering of venous pressures that drive symptomatology in patients with heart failure.
요약 맞춤 설정
AI로 다시 쓰기
인용 생성
소스 번역
다른 언어로
마인드맵 생성
소스 콘텐츠 기반
소스 방문
www.nature.com
Agonist antibody to guanylate cyclase receptor NPR1 regulates vascular tone - Nature
통계
Heart failure is a leading cause of morbidity and mortality.
Elevated intracardiac pressures and myocyte stretch in heart failure trigger the release of counter-regulatory natriuretic peptides.
Recombinant natriuretic peptide infusions were developed to treat heart failure but have been limited by a short duration of effect.
인용구
"Here we report that in a human genetic analysis of over 700,000 individuals, lifelong exposure to coding variants of the NPR1 gene is associated with changes in blood pressure and risk of heart failure."
"REGN5381, an allosteric agonist of NPR1, induces an active-like receptor conformation that results in haemodynamic effects preferentially on venous vasculature, including reductions in systolic blood pressure and venous pressure in animal models."
"In healthy human volunteers, REGN5381 produced the expected haemodynamic effects, reflecting reductions in venous pressures, without obvious changes in diuresis and natriuresis."
핵심 통찰 요약
by Michael E. D... 게시일 www.nature.com 09-11-2024
https://www.nature.com/articles/s41586-024-07903-1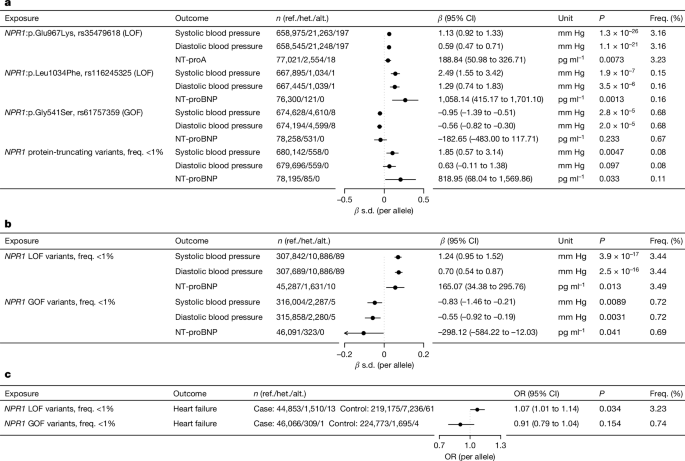
더 깊은 질문
What are the potential long-term safety and tolerability profiles of REGN5381 in patients with heart failure?
The long-term safety and tolerability profiles of REGN5381 in patients with heart failure will likely depend on several factors, including the drug's mechanism of action, its pharmacokinetics, and the patient population being treated. As an investigational monoclonal agonist antibody targeting the guanylate cyclase receptor NPR1, REGN5381 is designed to induce a conformational change in the receptor, leading to enhanced vasodilation and reductions in venous pressure.
In clinical trials, the safety profile will be assessed through monitoring adverse events, laboratory tests, and vital signs over extended periods. Given that REGN5381 primarily affects venous vasculature, it may have a lower risk of causing systemic hypotension compared to other heart failure therapies that exert broader hemodynamic effects. However, potential risks could include excessive vasodilation, leading to hypotension, or other off-target effects due to the modulation of the NPR1 pathway.
Long-term tolerability will also be evaluated by assessing the incidence of any immune responses to the monoclonal antibody, as well as the impact on renal function and electrolyte balance, particularly since heart failure patients often have comorbidities that complicate their treatment. Overall, ongoing clinical trials will provide critical data to establish the long-term safety and tolerability of REGN5381 in this vulnerable patient population.
How does the mechanism of action and hemodynamic effects of REGN5381 compare to other heart failure therapies targeting the natriuretic peptide system?
REGN5381 operates through a unique mechanism of action as an allosteric agonist of the NPR1 receptor, which is distinct from other heart failure therapies that target the natriuretic peptide system. Traditional therapies, such as recombinant natriuretic peptides, directly activate NPR1 but often have a short duration of effect, necessitating frequent dosing. In contrast, REGN5381 induces a more sustained receptor activation by stabilizing an active-like conformation of NPR1, potentially leading to prolonged hemodynamic effects.
The hemodynamic effects of REGN5381 are characterized by selective reductions in venous pressure and systolic blood pressure, which can alleviate symptoms of heart failure without significantly impacting diuresis and natriuresis. This selective action on the venous system may provide a therapeutic advantage over other agents that cause more generalized vasodilation, which can lead to adverse effects such as hypotension or renal impairment.
In summary, REGN5381's mechanism of action and hemodynamic profile may offer a novel approach to managing heart failure, particularly for patients who require long-lasting relief from venous congestion without the complications associated with broader vasodilatory therapies.
Could the selective venous effects of REGN5381 be leveraged to treat other conditions characterized by venous congestion, such as liver disease or chronic kidney disease?
Yes, the selective venous effects of REGN5381 could potentially be leveraged to treat other conditions characterized by venous congestion, such as liver disease and chronic kidney disease (CKD). In these conditions, elevated venous pressures can lead to significant morbidity, including ascites in liver disease and fluid overload in CKD.
By targeting the NPR1 receptor and inducing venodilation, REGN5381 may help to reduce venous pressure and alleviate symptoms associated with these conditions. For instance, in patients with cirrhosis, the reduction of portal venous pressure could mitigate complications such as variceal bleeding and ascites. Similarly, in CKD patients, lowering venous pressure may help manage fluid overload and improve overall cardiovascular health.
However, the application of REGN5381 in these contexts would require thorough investigation through clinical trials to assess efficacy, safety, and tolerability in populations with liver disease or CKD. The unique mechanism of REGN5381, focusing on venous vasodilation, presents an intriguing opportunity for expanding its therapeutic use beyond heart failure, potentially addressing significant unmet needs in the management of venous congestion-related disorders.
0
