Insights into G-Protein Activation by Cryo-EM
Grunnleggende konsepter
The author explores the mechanism of G-protein activation by utilizing time-resolved cryo-EM, revealing the conformational changes and events driving this process.
Sammendrag
Time-resolved cryo-EM was used to study the activation of G proteins by GPCRs. The study identified the structural changes that occur upon GTP binding, leading to the dissociation of the G protein from the receptor. This research provides a detailed understanding of the molecular events involved in GPCR signaling pathways.
Time-resolved cryo-EM of G-protein activation by a GPCR - Nature
Statistikk
"Twenty structures generated from sequential overlapping particle subsets along this trajectory provide a high-resolution description of the order of main events driving G-protein activation."
"Molecular dynamics simulations with late structures in the cryo-EM trajectory support enhanced ordering of GTP on closure of the α-helical domain against the nucleotide-bound Ras-homology domain."
Sitater
"Structural changes propagate from the nucleotide-binding pocket and extend through the GTPase domain, enacting alterations to Gα switch regions and the α5 helix."
"These findings highlight the potential of time-resolved cryo-EM as a tool for mechanistic dissection of GPCR signaling events."
Viktige innsikter hentet fra
by Maka... klokken www.nature.com 03-13-2024
https://www.nature.com/articles/s41586-024-07153-1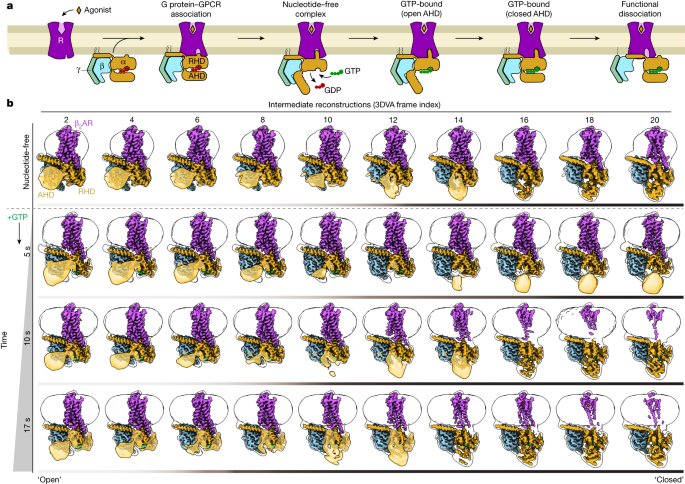
Dypere Spørsmål
How do these findings impact drug development targeting GPCRs
The findings from the time-resolved cryo-EM study provide crucial insights into the conformational changes and molecular events underlying G-protein activation by GPCRs. This detailed understanding of the structural dynamics involved in GPCR signaling can significantly impact drug development targeting GPCRs. By elucidating the specific mechanisms through which GPCRs interact with and activate heterotrimeric G proteins, researchers can design more precise and effective drugs that modulate these interactions. The high-resolution description of the order of events driving G-protein activation offers a roadmap for developing novel therapeutics that target key steps in this process, potentially leading to more potent and selective drugs with fewer side effects.
What are potential limitations or biases in using cryo-EM for studying protein interactions
While cryo-EM is a powerful technique for visualizing protein structures at near-atomic resolution, there are several potential limitations and biases associated with its use in studying protein interactions. One limitation is sample preparation variability, which can introduce artifacts or distortions in the final structures obtained through cryo-EM. Additionally, certain protein conformations or dynamic states may be challenging to capture using traditional cryo-EM techniques, leading to potential biases towards more stable conformations. Another limitation is the computational complexity involved in analyzing large datasets generated by time-resolved cryo-EM experiments, which may require sophisticated algorithms and significant computational resources.
How can understanding these molecular events lead to advancements in personalized medicine
Understanding the molecular events underlying GPCR signaling and protein interactions has profound implications for personalized medicine. Since many drugs target GPCRs due to their critical roles in various physiological processes, insights gained from studies like time-resolved cryo-EM can inform the development of precision therapies tailored to individual patients' genetic makeup or disease profiles. By deciphering how specific mutations or variations affect receptor-G protein interactions at a structural level, researchers can potentially identify new drug targets or optimize existing treatments for better efficacy and reduced adverse effects. This knowledge paves the way for personalized approaches where therapies are customized based on an individual's unique biological characteristics, ultimately improving treatment outcomes across diverse patient populations.
0
