Conformational Dynamics of the μ-Opioid Receptor Reveal Ligand-Specific Efficacy Modulation
The content discusses the molecular understanding of drug action on the μ-opioid receptor (μOR), which is an important target for pain management. Using advanced biophysical techniques, the researchers identified several conformations of the cytoplasmic face of the μOR that interconvert on different timescales. These conformations include a pre-activated conformation capable of G-protein binding and a fully activated conformation that markedly reduces GDP affinity within the ternary complex. The interaction of β-arrestin-1 with the μOR core binding site appears less specific and occurs with much lower affinity than the binding of Gi. The findings provide insights into how ligand-specific conformational changes of the μOR translate into a broad range of intrinsic efficacies at the transducer level, which can inform the development of better therapeutics.
Tilpass sammendrag
Omskriv med AI
Generer sitater
Oversett kilde
Til et annet språk
Generer tankekart
fra kildeinnhold
Besøk kilde
www.nature.com
Ligand efficacy modulates conformational dynamics of the µ-opioid receptor - Nature
Viktige innsikter hentet fra
by Jiawei Zhao,... klokken www.nature.com 04-10-2024
https://www.nature.com/articles/s41586-024-07295-2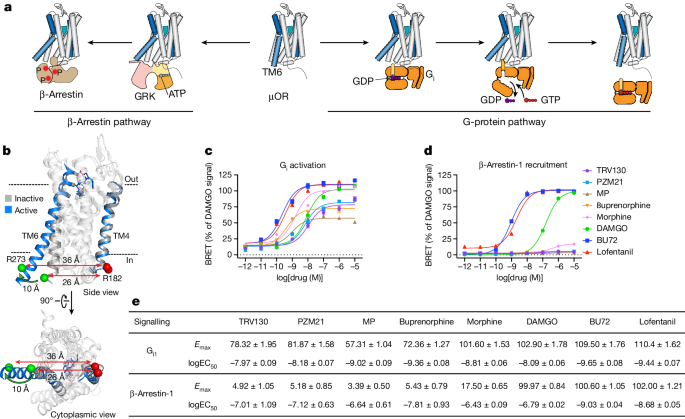
Dypere Spørsmål
