Multisensory Gamma Stimulation Enhances Amyloid Clearance in Alzheimer's Disease Mouse Model
Grunnleggende konsepter
Multisensory gamma stimulation enhances glymphatic clearance of amyloid in the brain, offering a potential therapeutic approach for Alzheimer's disease.
Sammendrag
The content discusses how multisensory gamma stimulation can promote the removal of amyloid in the brain, a key factor in Alzheimer's disease pathology. By inducing influx and efflux of cerebrospinal and interstitial fluids, this stimulation method shows promise in enhancing glymphatic clearance. Additionally, the involvement of vasoactive intestinal peptide interneurons in regulating arterial pulsatility to facilitate amyloid removal highlights novel mechanisms for targeting Alzheimer's disease pathology.
Multisensory gamma stimulation promotes glymphatic clearance of amyloid - Nature
Statistikk
Noninvasive 40 Hz stimulation promotes neural activity and attenuates pathology in mouse models of Alzheimer’s disease.
Multisensory gamma stimulation promotes the influx of cerebrospinal fluid and efflux of interstitial fluid in the cortex.
Inhibiting glymphatic clearance abolishes the removal of amyloid by multisensory 40 Hz stimulation.
Vasoactive intestinal peptide interneurons regulate arterial pulsatility to facilitate glymphatic clearance.
Sitater
Viktige innsikter hentet fra
by Mitchell H. ... klokken www.nature.com 02-28-2024
https://www.nature.com/articles/s41586-024-07132-6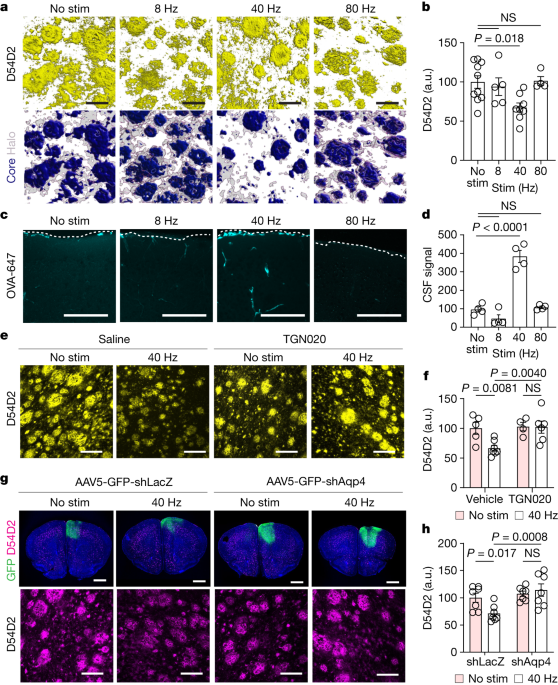
Dypere Spørsmål
How can multisensory gamma stimulation be translated into clinical applications for treating Alzheimer's disease
Multisensory gamma stimulation has shown promising results in promoting glymphatic clearance of amyloid in mouse models of Alzheimer's disease. To translate this into clinical applications, further research and development are needed to optimize the stimulation parameters for human use. Clinical trials can be designed to assess the safety and efficacy of multisensory gamma stimulation in individuals with Alzheimer's disease. This could involve noninvasive techniques such as transcranial magnetic or electrical stimulation targeted at specific brain regions implicated in amyloid clearance. Monitoring tools like imaging modalities can track changes in glymphatic function and amyloid levels before and after treatment. Personalized approaches may also be explored by tailoring the stimulation protocols based on individual variations in brain anatomy and pathology.
What are potential limitations or challenges associated with targeting the glymphatic system for amyloid clearance
While targeting the glymphatic system for amyloid clearance holds promise, there are several potential limitations and challenges that need to be addressed. One limitation is the complexity of the glymphatic system itself, which involves intricate interactions between different cell types, molecules, and fluid dynamics within the brain. Manipulating this system without disrupting normal physiological processes poses a challenge. Another concern is ensuring specificity in targeting only harmful substances like amyloid while preserving essential molecules involved in normal brain function. Additionally, variability among individuals regarding glymphatic efficiency due to factors like age or comorbidities may affect treatment outcomes. Strategies to enhance glymphatic function without causing unintended side effects will require careful consideration.
How might understanding neuropeptide signaling pathways lead to new therapeutic interventions beyond Alzheimer's disease
Understanding neuropeptide signaling pathways opens up new avenues for therapeutic interventions beyond Alzheimer's disease. Neuropeptides play crucial roles in modulating various physiological functions throughout the body, including neurotransmission, pain perception, stress response, and immune regulation. By elucidating how neuropeptides influence processes like arterial pulsatility linked to glymphatic clearance, researchers can explore novel targets for treating conditions beyond neurodegenerative diseases. For instance, targeting neuropeptide receptors involved in regulating vascular tone could lead to innovative therapies for cardiovascular disorders or migraine headaches by modulating blood flow dynamics through similar mechanisms identified in glymphatic modulation studies related to Alzheimer's disease.
0
