Molecular Mesh Buildup Around Hunger Neurons Drives Obesity in Mice
Główne pojęcia
Buildup of a molecular meshwork around neurons in the arcuate nucleus of the hypothalamus blocks insulin signaling, leading to increased food intake, fat accumulation, and metabolic problems in obese mice.
Streszczenie
The article discusses how obesity is driven by a buildup of a molecular meshwork called the extracellular matrix around neurons in the arcuate nucleus of the hypothalamus (ARC). This meshwork prevents insulin, which is secreted by the pancreas after a meal, from reaching its receptor on these neurons.
Normally, insulin signaling in the ARC helps regulate food intake, fat burning, and energy expenditure. However, when neurons lose sensitivity to insulin due to this extracellular matrix buildup, the mice start eating more, accumulating more fat, and developing metabolic issues.
The researchers found that by freeing the neurons from this molecular meshwork, they were able to restore insulin signaling and dramatically reduce the body weight of the obese mice. This suggests that targeting the extracellular matrix around ARC neurons could be a potential therapeutic approach for treating obesity and related metabolic disorders.
Obesity is driven by a build-up of molecular mesh around hunger neurons
Statystyki
After eating a meal, the concentration of glucose in the blood rises and triggers β-cells in the pancreas to secrete more of the hormone insulin.
If neurons in the ARC lose sensitivity to insulin, we start to eat more, accumulate fat and develop problems with our metabolic health.
Freeing neurons from this meshwork restores insulin signalling and drastically reduces body weight.
Cytaty
"A build-up of a molecular meshwork called the extracellular matrix around neurons stops insulin from reaching its receptor, driving insulin resistance in the brains of obese mice."
"Freeing neurons from this meshwork restores insulin signalling and drastically reduces body weight."
Kluczowe wnioski z
by Alexander Di... o www.nature.com 09-18-2024
https://www.nature.com/articles/d41586-024-02325-5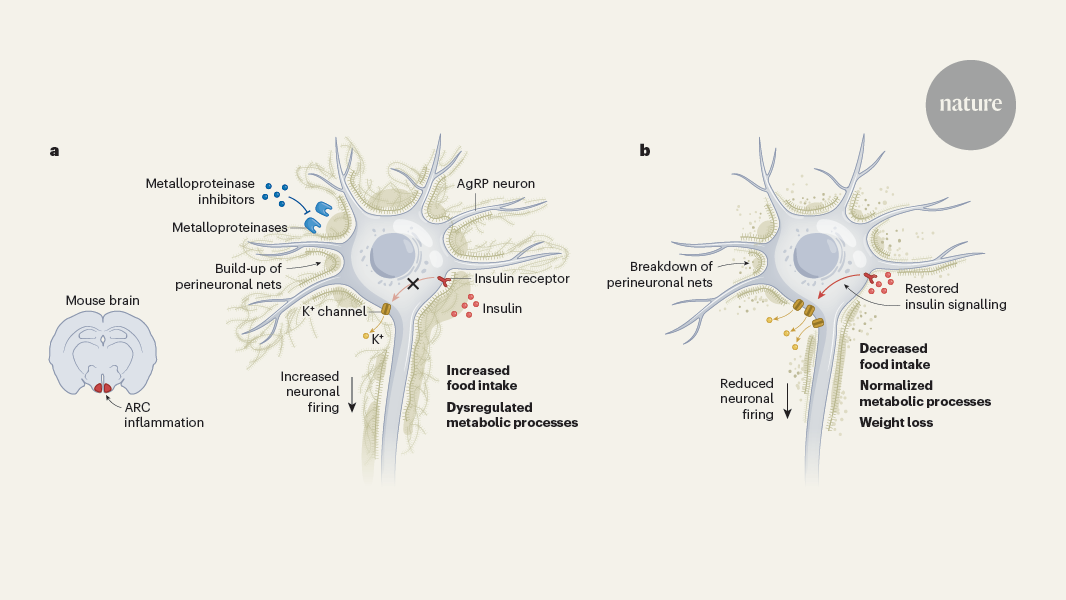
Głębsze pytania
What other factors, besides the extracellular matrix buildup, could contribute to the development of insulin resistance in the ARC neurons of obese individuals?
In addition to the extracellular matrix (ECM) buildup, several other factors can contribute to the development of insulin resistance in the arcuate nucleus of the hypothalamus (ARC) neurons in obese individuals. One significant factor is chronic inflammation, which is often associated with obesity. Adipose tissue in obese individuals secretes pro-inflammatory cytokines that can impair insulin signaling pathways, leading to reduced sensitivity of neurons to insulin. Additionally, alterations in lipid metabolism, such as increased levels of free fatty acids, can also interfere with insulin signaling in the brain.
Moreover, hormonal changes, including elevated levels of leptin and decreased levels of adiponectin, can disrupt the normal functioning of ARC neurons. Leptin resistance, a common condition in obesity, can further exacerbate the inability of these neurons to respond to insulin effectively. Lastly, lifestyle factors such as poor diet, lack of physical activity, and sleep disturbances can also play a role in the development of insulin resistance by affecting metabolic health and neuronal function.
How might the findings from this study on mice translate to the treatment of obesity and metabolic disorders in humans?
The findings from the study by Beddows et al. suggest a promising avenue for treating obesity and metabolic disorders in humans by targeting the extracellular matrix (ECM) surrounding ARC neurons. If the ECM is indeed a barrier to insulin signaling, strategies aimed at reducing ECM buildup or enhancing its remodeling could restore insulin sensitivity in the hypothalamus.
Potential therapeutic approaches could include the development of drugs that specifically target the components of the ECM or the signaling pathways involved in its formation. Additionally, lifestyle interventions that promote weight loss and reduce inflammation may also help in alleviating ECM-related insulin resistance.
Furthermore, understanding the mechanisms by which ECM influences neuronal function could lead to novel treatments that not only address obesity but also improve metabolic health, potentially reducing the risk of associated conditions such as type 2 diabetes and cardiovascular diseases. Translating these findings into clinical practice will require rigorous testing in human trials to evaluate safety and efficacy.
Could targeting the extracellular matrix around ARC neurons have broader implications for understanding and treating other neurological or psychiatric disorders associated with disrupted hypothalamic function?
Yes, targeting the extracellular matrix (ECM) around ARC neurons could have broader implications for understanding and treating various neurological and psychiatric disorders associated with disrupted hypothalamic function. The hypothalamus plays a critical role in regulating numerous physiological processes, including appetite, energy balance, and stress responses. Disruptions in these processes can lead to conditions such as depression, anxiety, and eating disorders.
By investigating the role of the ECM in the hypothalamus, researchers may uncover new insights into how structural changes in the brain contribute to these disorders. For instance, if ECM alterations are found to affect neuronal signaling in the hypothalamus, it could lead to the development of targeted therapies that restore normal function and alleviate symptoms.
Moreover, since the ECM is involved in neuroplasticity and neuronal communication, understanding its role could also provide insights into neurodegenerative diseases where hypothalamic function is compromised. Overall, targeting the ECM may open new therapeutic avenues not only for obesity and metabolic disorders but also for a range of neurological and psychiatric conditions linked to hypothalamic dysfunction.
0
