insight - Computational Biology - # Structural Mechanism of RNA-Guided DNA Recombination in Prokaryotic Insertion Sequence Elements
Structural Mechanism of Bridge RNA-Guided Recombination in Insertion Sequence Elements
Conceitos essenciais
Insertion sequence (IS) elements in prokaryotic genomes utilize a recombinase and a non-coding bridge RNA (bRNA) to confer modular specificity for target DNA and donor DNA, enabling programmable DNA recombination.
Resumo
The content describes the structural mechanism of RNA-guided DNA recombination in prokaryotic insertion sequence (IS) elements. Key insights:
IS110 family elements encode a recombinase and a non-coding bridge RNA (bRNA) that confers modular specificity for target DNA and donor DNA through two programmable loops.
The cryo-electron microscopy structures of the IS110 recombinase in complex with its bRNA, target DNA, and donor DNA reveal the three-stage recombination reaction cycle:
The IS110 synaptic complex comprises two recombinase dimers, with one dimer binding to the target-binding loop of the bRNA and the target DNA, and the other dimer coordinating the bRNA donor-binding loop and the donor DNA.
A composite RuvC–Tnp active site is formed, spanning the two dimers and positioning the catalytic serine residues adjacent to the recombination sites in both target and donor DNA.
The top strands of target and donor DNA are cleaved at the composite active sites, forming covalent 5'-phosphoserine intermediates. The cleaved DNA strands are then exchanged and religated to create a Holliday junction intermediate, which is subsequently resolved by cleavage of the bottom strands.
This study reveals the mechanism by which a bispecific RNA confers target and donor DNA specificity to IS110 recombinases for programmable DNA recombination.
Structural mechanism of bridge RNA-guided recombination - Nature
Estatísticas
Insertion sequence (IS) elements are the simplest autonomous transposable elements found in prokaryotic genomes.
The IS110 recombinase and its bridge RNA (bRNA) confer modular specificity for target DNA and donor DNA through two programmable loops.
Citações
"We recently discovered that IS110 family elements encode a recombinase and a non-coding bridge RNA (bRNA) that confers modular specificity for target DNA and donor DNA through two programmable loops."
"A comparison of the three structures revealed that (1) the top strands of target and donor DNA are cleaved at the composite active sites to form covalent 5′-phosphoserine intermediates, (2) the cleaved DNA strands are exchanged and religated to create a Holliday junction intermediate, and (3) this intermediate is subsequently resolved by cleavage of the bottom strands."
Principais Insights Extraídos De
by Masahiro Hir... às www.nature.com 06-26-2024
https://www.nature.com/articles/s41586-024-07570-2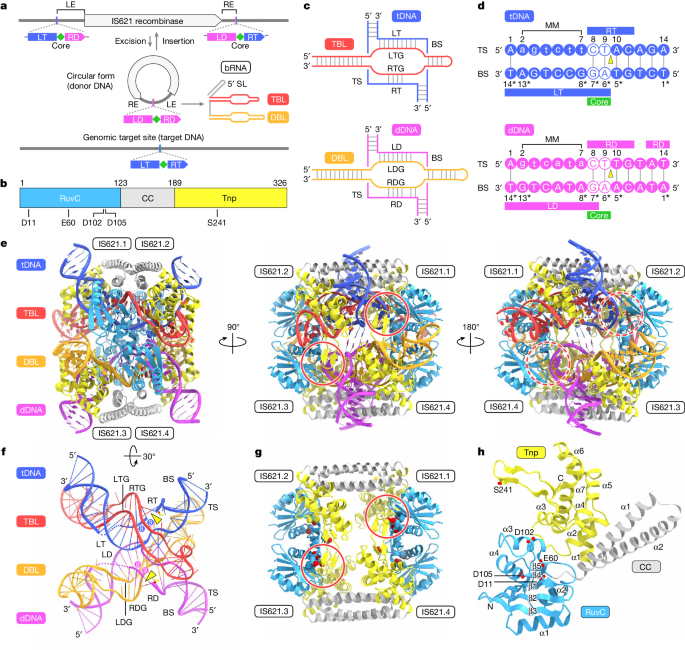
Perguntas Mais Profundas
How might the insights from this study on RNA-guided DNA recombination in prokaryotic IS elements be applied to develop novel genome engineering tools?
The study on RNA-guided DNA recombination in prokaryotic IS elements provides valuable insights into the structural mechanism of bridge RNA (bRNA)-guided recombination. These insights could be applied to develop novel genome engineering tools by harnessing the modular specificity conferred by bRNA. By understanding how the bRNA interacts with target DNA and donor DNA through programmable loops, researchers can design synthetic RNA molecules that guide specific DNA recombination events in a controlled manner. This knowledge can be leveraged to create customizable genome editing tools that enable precise modifications in prokaryotic genomes, offering a powerful platform for genetic engineering applications.
What are the potential limitations or challenges in translating this mechanism to eukaryotic systems, and how could they be addressed?
Translating the mechanism of RNA-guided DNA recombination from prokaryotic IS elements to eukaryotic systems poses several potential limitations and challenges. One major challenge is the structural and functional differences between prokaryotic and eukaryotic genomes, which may affect the efficiency and specificity of the recombination process. Eukaryotic genomes are more complex, with additional regulatory elements and chromatin organization that could interfere with the binding and activity of the recombinase-bRNA complex. Additionally, the delivery of the recombinase-bRNA complex into eukaryotic cells and targeting specific genomic regions may be challenging due to the presence of nuclear membranes and other cellular barriers.
To address these challenges, researchers could explore strategies to optimize the design of the recombinase-bRNA complex for compatibility with eukaryotic genomes. This may involve modifying the structure of the bRNA or the recombinase to enhance their stability and activity in eukaryotic cells. Additionally, developing efficient delivery methods, such as viral vectors or nanoparticles, could facilitate the targeted delivery of the recombinase-bRNA complex into eukaryotic cells. By overcoming these limitations, the mechanism of RNA-guided DNA recombination could potentially be translated to eukaryotic systems for genome engineering applications.
Given the modular specificity conferred by the bRNA, could this system be adapted to target and recombine specific genomic regions in a programmable manner, and what would be the implications for applications in biotechnology and medicine?
The modular specificity conferred by the bridge RNA (bRNA) in prokaryotic IS elements presents a promising opportunity to adapt this system for targeting and recombining specific genomic regions in a programmable manner. By designing synthetic bRNAs with customized target-binding and donor-binding loops, researchers can precisely direct the recombination of DNA sequences at desired genomic loci. This programmable approach to DNA recombination could revolutionize genome editing technologies by enabling the precise modification of genes associated with genetic disorders, cancer, or other diseases.
In biotechnology, the ability to target and recombine specific genomic regions programmably could lead to the development of advanced gene editing tools for creating genetically modified organisms, engineering microbial strains for bioproduction, or designing novel therapeutic interventions. In medicine, this technology could offer new possibilities for gene therapy, personalized medicine, and targeted treatments for genetic diseases. By harnessing the modular specificity of bRNA-guided recombination, researchers can unlock a wide range of applications with significant implications for both biotechnology and medicine.
0
