Harnessing Type 2 Immunity to Revitalize Exhausted CD8+ T Cells and Enhance Cancer Immunotherapy
Conceitos essenciais
Type 2 cytokine Fc-IL-4 can directly act on CD8+ T cells to enrich and reinvigorate terminally exhausted CD8+ T cells, thereby enhancing the efficacy of type 1 immunity-based cancer immunotherapies.
Resumo
The article explores the potential of harnessing type 2 immunity, specifically the interleukin-4 (IL-4) cytokine, to improve the efficacy of cancer immunotherapy. Current cancer immunotherapy primarily focuses on eliciting type 1 immune responses, but long-term complete remission remains uncommon.
The researchers demonstrate that an IL-4 fusion protein (Fc-IL-4), a typical type 2 cytokine, can directly act on CD8+ T cells and enrich functional terminally exhausted CD8+ T (CD8+ TTE) cells within the tumor. Consequently, Fc-IL-4 enhances the antitumor efficacy of type 1 immunity-centric adoptive T cell transfer or immune checkpoint blockade therapies, leading to durable remission across several tumor models.
Mechanistically, the authors found that Fc-IL-4 signals through both the STAT6 and mTOR pathways, augmenting the glycolytic metabolism and NAD concentration of CD8+ TTE cells in a lactate dehydrogenase A-dependent manner. This metabolic modulation is crucial for reinvigorating intratumoral CD8+ TTE cells.
The study highlights Fc-IL-4 as a potent type 2 cytokine-based immunotherapy that can synergize effectively with type 1 immunity to elicit long-lasting responses against cancer. It provides insights into the potential of integrating type 2 immune factors to advance next-generation cancer immunotherapy.
The type 2 cytokine Fc–IL-4 revitalizes exhausted CD8+ T cells against cancer - Nature
Estatísticas
Current cancer immunotherapy predominately focuses on eliciting type 1 immune responses fighting cancer; however, long-term complete remission remains uncommon.
Fc-IL-4 enhances antitumour efficacy of type 1 immunity-centric adoptive T cell transfer or immune checkpoint blockade therapies and induces durable remission across several syngeneic and xenograft tumour models.
Fc-IL-4 signals through both STAT6 and mTOR pathways, augmenting the glycolytic metabolism and the NAD concentration of CD8+ TTE cells in a lactate dehydrogenase A-dependent manner.
Citações
"A pivotal question arises as to whether type 2 immunity can be orchestrated alongside type 1-centric immunotherapy to achieve enduring response against cancer."
"These findings underscore Fc–IL-4 as a potent type 2 cytokine-based immunotherapy that synergizes effectively with type 1 immunity to elicit long-lasting responses against cancer."
Principais Insights Extraídos De
by Bing Feng,Zh... às www.nature.com 09-25-2024
https://www.nature.com/articles/s41586-024-07962-4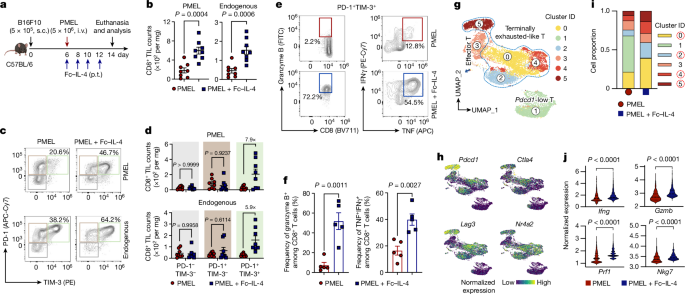
Perguntas Mais Profundas
How can the synergistic effects of type 1 and type 2 immunity be further optimized to achieve even more durable and comprehensive cancer remission?
To further optimize the synergistic effects of type 1 and type 2 immunity for achieving durable and comprehensive cancer remission, several strategies can be considered. First, a deeper understanding of the specific cytokine profiles and immune cell interactions involved in both type 1 and type 2 responses is essential. This could involve the identification of additional type 2 cytokines that may enhance the functionality of CD8+ T cells when used in conjunction with type 1 therapies, such as immune checkpoint inhibitors or adoptive T cell transfer.
Second, the timing and dosing of Fc–IL-4 administration in relation to type 1 therapies could be fine-tuned. For instance, administering Fc–IL-4 at strategic points during the treatment regimen may maximize its effects on CD8+ TTE cells, potentially leading to improved metabolic reprogramming and enhanced antitumor activity.
Third, combination therapies that include other immune modulators, such as those targeting regulatory T cells or myeloid-derived suppressor cells, could further enhance the efficacy of the type 1/type 2 immune response synergy. This multi-faceted approach may help to create a more favorable tumor microenvironment, allowing for sustained immune activation and improved tumor control.
Lastly, personalized medicine approaches that tailor immunotherapy based on the individual patient's tumor microenvironment and immune profile could lead to more effective combinations of type 1 and type 2 immune strategies, ultimately resulting in longer-lasting remissions.
What are the potential limitations or drawbacks of using Fc-IL-4 or other type 2 cytokine-based therapies in combination with type 1 immunity-centric approaches?
While Fc–IL-4 and other type 2 cytokine-based therapies show promise in enhancing type 1 immunity against cancer, there are potential limitations and drawbacks to consider. One significant concern is the risk of promoting an overly robust type 2 immune response, which could lead to immune dysregulation or adverse effects, such as increased tumor progression in certain contexts. Type 2 cytokines can sometimes support tumor growth by fostering an immunosuppressive microenvironment, particularly if the tumor has mechanisms to exploit these signals.
Additionally, the metabolic reprogramming induced by Fc–IL-4 may not be uniformly beneficial across all tumor types or stages. Some tumors may adapt to the enhanced glycolytic metabolism of CD8+ TTE cells, leading to resistance against immunotherapy. Furthermore, the reliance on specific signaling pathways, such as STAT6 and mTOR, raises concerns about potential off-target effects or toxicity associated with prolonged exposure to type 2 cytokines.
Moreover, the development of neutralizing antibodies against Fc–IL-4 or other type 2 cytokines could diminish their therapeutic efficacy over time, necessitating careful monitoring and potential adjustments in treatment strategies. Lastly, the complexity of the immune system means that individual patient variability in response to type 2 cytokine therapy could lead to inconsistent outcomes, highlighting the need for personalized approaches in cancer immunotherapy.
What other metabolic pathways or cellular mechanisms might be involved in the reinvigoration of exhausted CD8+ T cells by Fc-IL-4, and how could these insights be leveraged to develop more effective cancer immunotherapies?
In addition to the mTOR and glycolytic pathways already implicated in the reinvigoration of exhausted CD8+ T cells by Fc–IL-4, several other metabolic pathways and cellular mechanisms may play critical roles. For instance, the activation of the AMP-activated protein kinase (AMPK) pathway could enhance energy metabolism and promote T cell survival and function. AMPK activation is known to improve mitochondrial biogenesis and fatty acid oxidation, which could further support the metabolic needs of CD8+ TTE cells.
Additionally, the role of the tricarboxylic acid (TCA) cycle and oxidative phosphorylation in T cell metabolism warrants investigation. Enhancing these pathways may provide alternative energy sources for CD8+ T cells, potentially improving their persistence and functionality in the tumor microenvironment.
Furthermore, the modulation of epigenetic factors that influence T cell exhaustion and differentiation could be another avenue for enhancing the efficacy of Fc–IL-4. For example, targeting histone deacetylases (HDACs) or DNA methyltransferases may help to reverse the epigenetic changes associated with T cell exhaustion, thereby restoring their antitumor capabilities.
Leveraging these insights into metabolic pathways and cellular mechanisms could lead to the development of combination therapies that not only include Fc–IL-4 but also target these additional pathways. Such strategies could enhance the reinvigoration of exhausted CD8+ T cells, improve their metabolic fitness, and ultimately lead to more effective and durable cancer immunotherapies.
0
