Interferon Disrupts AHR-JUN Axis to Induce CXCL13-Producing T Cells in Systemic Lupus Erythematosus
The article investigates the regulation of CXCL13-producing T cells in systemic lupus erythematosus (SLE), a prototypical autoimmune disease driven by pathological T cell-B cell interactions. The authors identify an imbalance in CD4+ T cell phenotypes in SLE patients, with an expansion of PD-1+/ICOS+ CXCL13+ T cells and a reduction of CD96hi IL-22+ T cells.
Using CRISPR screens, the authors find that the aryl hydrocarbon receptor (AHR) is a potent negative regulator of CXCL13 production by human CD4+ T cells. Transcriptomic, epigenetic, and functional studies demonstrate that AHR coordinates with the AP-1 family member JUN to prevent CXCL13+ T follicular helper (TFH) and T peripheral helper (TPH) cell differentiation and promote an IL-22+ T helper 22 (TH22) phenotype.
Importantly, the authors show that type I interferon, a pathogenic driver of SLE, opposes the AHR-JUN axis to promote T cell production of CXCL13. These findings place CXCL13+ TFH/TPH cells on a polarization axis opposite from TH22 cells and reveal AHR, JUN, and interferon as key regulators of these divergent T cell states in the context of SLE.
Customize Summary
Rewrite with AI
Generate Citations
Translate Source
To Another Language
Generate MindMap
from source content
Visit Source
www.nature.com
Interferon subverts an AHR–JUN axis to promote CXCL13+ T cells in lupus - Nature
Principais Insights Extraídos De
by Calvin Law,V... às www.nature.com 07-10-2024
https://www.nature.com/articles/s41586-024-07627-2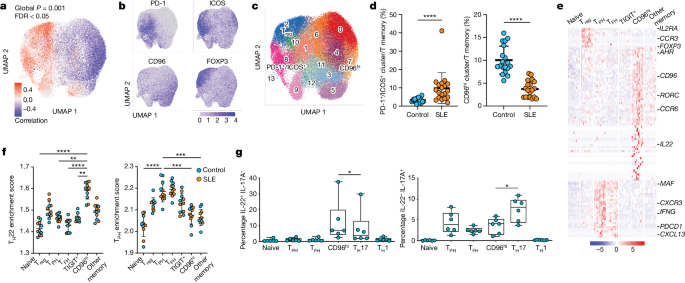
Perguntas Mais Profundas
