Mitochondrial Transfer Enhances Endothelial Cell Engraftment in Ischemic Tissues through Mitophagy
Centrala begrepp
Mesenchymal stromal cells (MSCs) transfer mitochondria to endothelial cells (ECs) under cellular stress, enabling enhanced EC engraftment and vessel formation in ischemic tissues without the need for co-transplanting supporting cells.
Sammanfattning
The article investigates the mechanisms underlying the ability of mesenchymal stromal cells (MSCs) to facilitate the engraftment of transplanted endothelial cells (ECs) in ischemic tissues. The key findings are:
Under cellular stress, MSCs transfer mitochondria to ECs through tunneling nanotubes. Blocking this mitochondrial transfer impairs EC engraftment.
Artificially transplanting mitochondria to ECs can transiently enhance their bioenergetics and enable them to form functional vessels in ischemic tissues without the support of MSCs.
The exogenous mitochondria do not integrate into the endogenous EC mitochondrial pool, but instead trigger mitophagy (selective degradation of mitochondria) after internalization.
The enhanced engraftment ability of ECs with exogenous mitochondria is dependent on the PINK1-Parkin pathway, which regulates mitophagy.
The authors conclude that the mechanism of mitochondrial transfer from MSCs to ECs, and the subsequent induction of mitophagy, underlies the ability of MSCs to support EC engraftment. This offers a potential new approach for vascular cell therapy that does not require co-transplantation of supporting cells.
Mitochondrial transfer mediates endothelial cell engraftment through mitophagy - Nature
Statistik
Ischaemic diseases such as critical limb ischaemia and myocardial infarction affect millions of people worldwide.
Citat
"Transplanting endothelial cells (ECs) is a promising therapy in vascular medicine, but engrafting ECs typically necessitates co-transplanting perivascular supporting cells such as mesenchymal stromal cells (MSCs), which makes clinical implementation complicated."
"We devised a strategy to artificially transplant mitochondria, transiently enhancing EC bioenergetics and enabling them to form functional vessels in ischaemic tissues without the support of MSCs."
Viktiga insikter från
by Ruei-Zeng Li... på www.nature.com 05-01-2024
https://www.nature.com/articles/s41586-024-07340-0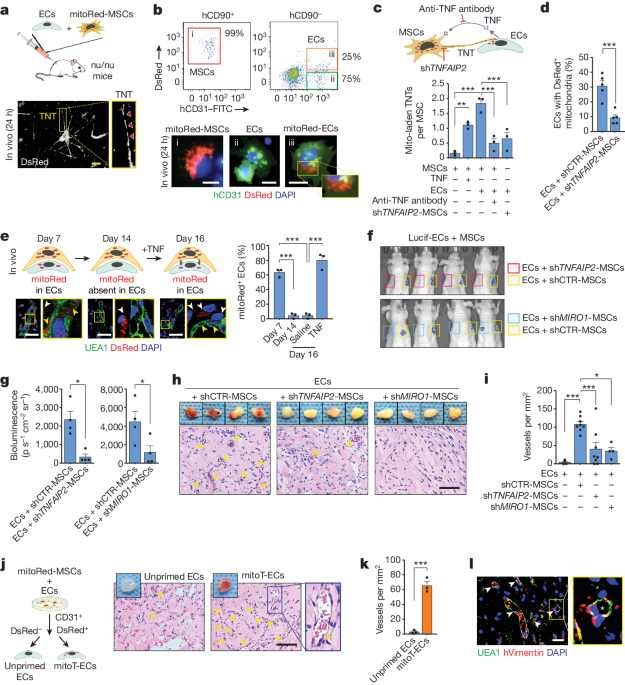
Djupare frågor
What are the potential long-term effects of the artificially transplanted mitochondria on the engrafted endothelial cells and the surrounding tissue?
The artificially transplanted mitochondria could have several long-term effects on the engrafted endothelial cells and the surrounding tissue. Firstly, the enhanced bioenergetics provided by the exogenous mitochondria could lead to sustained improvement in the functionality of the engrafted endothelial cells, promoting better vascularization and tissue repair over time. Additionally, the activation of mitophagy triggered by the transplanted mitochondria could help maintain mitochondrial quality within the endothelial cells, preventing the accumulation of damaged mitochondria that could otherwise lead to cellular dysfunction or apoptosis. This process may contribute to the long-term viability and functionality of the engrafted endothelial cells, ultimately supporting tissue regeneration and repair in ischaemic conditions.
How can the mitochondrial transfer and mitophagy mechanisms be further optimized to improve the efficiency and scalability of this approach for clinical applications?
To enhance the efficiency and scalability of the mitochondrial transfer and mitophagy mechanisms for clinical applications, several strategies can be considered. Firstly, optimizing the process of mitochondrial transfer through tunnelling nanotubes between mesenchymal stromal cells (MSCs) and endothelial cells (ECs) could be achieved by modulating the formation and stability of these nanotubes. This could involve the use of specific signaling molecules or factors that promote the formation of functional nanotubes for efficient mitochondrial transfer. Additionally, enhancing the rate of mitophagy in ECs following the internalization of exogenous mitochondria could be crucial for maintaining cellular homeostasis and maximizing the benefits of mitochondrial transplantation. This could be achieved by targeting key regulators of mitophagy pathways, such as the PINK1-Parkin pathway, to ensure efficient clearance of damaged mitochondria and promote the integration of functional exogenous mitochondria into the cellular pool. Moreover, exploring novel delivery methods for exogenous mitochondria, such as nanocarriers or engineered vesicles, could improve the targeted delivery and uptake of mitochondria by ECs, further enhancing the therapeutic potential of this approach for clinical applications.
What other cellular or molecular pathways might be involved in the interplay between mesenchymal stromal cells and endothelial cells during the engraftment process?
In addition to the mitochondrial transfer and mitophagy mechanisms, several other cellular or molecular pathways may play a role in the interplay between mesenchymal stromal cells (MSCs) and endothelial cells (ECs) during the engraftment process. One such pathway is the Notch signaling pathway, which has been implicated in regulating the interaction between MSCs and ECs and promoting angiogenesis and vascular development. Notch signaling mediates cell-cell communication and can influence the differentiation and function of both MSCs and ECs, thereby modulating their engraftment potential and vascularization capacity. Additionally, the Wnt signaling pathway, known for its role in stem cell maintenance and tissue regeneration, may also contribute to the crosstalk between MSCs and ECs during the engraftment process. Activation of Wnt signaling in MSCs could promote their paracrine signaling effects on ECs, enhancing their survival, proliferation, and engraftment in ischaemic tissues. Furthermore, the PI3K-Akt signaling pathway, involved in cell growth, survival, and metabolism, may be crucial for mediating the effects of mitochondrial transfer on EC bioenergetics and function. Activation of PI3K-Akt signaling in response to mitochondrial transplantation could enhance the regenerative potential of engrafted ECs and promote vascular repair in ischaemic conditions.
0
