Structural Insights into the Calcium-Sensing Receptor's Ability to Activate Multiple G-Protein Subtypes
แนวคิดหลัก
The calcium-sensing receptor (CaSR) can activate multiple G-protein subtypes through a common binding mode, facilitated by the receptor's dimeric structure and the flexibility of its intracellular loops.
บทคัดย่อ
The content discusses the structural basis for the calcium-sensing receptor's (CaSR) ability to signal through various G-protein subtypes, including Gq, Gi, and Gs. Key insights:
The homodimeric CaSR couples to a single G protein through a common binding mode, where the C-terminal helix of each Gα subunit binds to a shallow pocket formed by the receptor's intracellular loops, transmembrane helix 3, and C-terminal region.
G-protein binding expands the transmembrane dimer interface, which is further stabilized by phospholipid. The restraint imposed by the receptor dimer, combined with the flexibility of intracellular loop 2 (ICL2), enables G-protein activation by facilitating the conformational transition of Gα.
A single Gα residue determines the selectivity between Gq/Gs and Gi subtypes, while the length and flexibility of ICL2 allow CaSR to bind all three Gα subtypes, conferring the capacity for promiscuous G-protein coupling.
The functional pleiotropy of CaSR, which mediates diverse cellular processes beyond calcium homeostasis, is attributed to its ability to signal through multiple G-protein pathways.
Promiscuous G-protein activation by the calcium-sensing receptor - Nature
สถิติ
The calcium-sensing receptor (CaSR) can detect fluctuations in extracellular Ca2+ concentration and maintain Ca2+ homeostasis.
CaSR can signal through several G-protein subtypes, including Gq, Gi, and Gs.
The CaSR-G-protein complexes studied involve a single G protein binding to the homodimeric CaSR.
G-protein binding expands the transmembrane dimer interface of CaSR, which is further stabilized by phospholipid.
A single Gα residue determines the selectivity between Gq/Gs and Gi subtypes.
คำพูด
"The functional pleiotropy of CaSR arises in part from its ability to signal through several G-protein subtypes."
"We found that the homodimeric CaSR of each complex couples to a single G protein through a common mode."
"The restraint imposed by the receptor dimer, in combination with ICL2, enables G-protein activation by facilitating conformational transition of Gα."
ข้อมูลเชิงลึกที่สำคัญจาก
by Hao Zuo,Jins... ที่ www.nature.com 04-17-2024
https://www.nature.com/articles/s41586-024-07331-1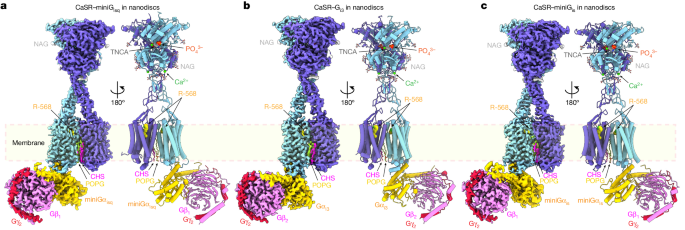
สอบถามเพิ่มเติม
How do the structural features of CaSR, such as the dimer interface and intracellular loops, contribute to its ability to regulate diverse cellular processes beyond calcium homeostasis?
The structural features of CaSR play a crucial role in its ability to regulate diverse cellular processes beyond calcium homeostasis. The dimer interface of CaSR, formed by the homodimeric arrangement of the receptor, allows for the coupling of a single G protein through a common mode. This interaction involves the binding of the C-terminal helix of each Gα subunit to a shallow pocket on CaSR, created by the intracellular loops (ICL1–ICL3), transmembrane helix 3, and an ordered C-terminal region. The expansion of the transmembrane dimer interface upon G-protein binding, further stabilized by phospholipids, facilitates the conformational transition of Gα, enabling G-protein activation. Additionally, the flexibility and length of ICL2 in CaSR allow it to bind to G proteins from different subfamilies, leading to promiscuous G-protein coupling. This promiscuity in G-protein activation contributes to the functional pleiotropy of CaSR, allowing it to mediate various cellular processes beyond calcium homeostasis.
What are the potential implications of CaSR's promiscuous G-protein coupling for the development of targeted therapies for conditions associated with dysregulated CaSR signaling?
The promiscuous G-protein coupling of CaSR has significant implications for the development of targeted therapies for conditions associated with dysregulated CaSR signaling. Understanding the structural mechanisms that enable CaSR to interact with multiple G-protein subtypes provides insights into potential therapeutic targets for modulating CaSR activity. By targeting specific residues or regions involved in G-protein binding and activation, researchers may be able to develop drugs that selectively modulate CaSR signaling pathways. This targeted approach could lead to the development of novel therapies for conditions where dysregulated CaSR signaling plays a role, such as hyperparathyroidism, osteoporosis, and certain cancers. By specifically targeting the promiscuous G-protein coupling of CaSR, tailored treatments could be designed to restore normal cellular function and address disease pathology.
How might the insights into CaSR's structural mechanisms inform the design of other G-protein-coupled receptors with the ability to activate multiple signaling pathways?
Insights into CaSR's structural mechanisms can provide valuable information for the design of other G-protein-coupled receptors (GPCRs) with the ability to activate multiple signaling pathways. Understanding how CaSR interacts with different G-protein subtypes through specific structural features, such as the dimer interface and intracellular loops, can guide the engineering of GPCRs with similar promiscuous G-protein coupling capabilities. By incorporating key structural elements identified in CaSR, such as the binding pocket for Gα subunits and the flexible ICL2 region, researchers can design novel GPCRs that have the capacity to activate diverse signaling pathways. This knowledge can be leveraged to create engineered receptors tailored for specific therapeutic purposes, allowing for the development of targeted drugs that modulate multiple signaling cascades through a single receptor. By applying the structural insights gained from CaSR to the design of other GPCRs, researchers can expand the repertoire of potential drug targets and enhance the efficacy of future pharmacological interventions.
0
