Understanding the Degradation of Nuclear cGAS by CRL5–SPSB3 Ubiquitin Ligase
核心概念
The author argues that the ubiquitin proteasomal system degrades nuclear cGAS in cycling cells through the action of SPSB3, shedding light on a crucial regulatory mechanism for innate immune responses.
摘要
Cyclic GMP-AMP synthase (cGAS) plays a vital role in sensing aberrant DNA and initiating immune responses. The study reveals that nuclear cGAS is degraded by the UPS, specifically through SPSB3, impacting cell physiology and immune signaling against DNA viruses.
The CRL5–SPSB3 ubiquitin ligase targets nuclear cGAS for degradation - Nature
統計資料
Cyclic GMP-AMP synthase (cGAS) senses aberrant DNA during infection, cancer, and inflammatory disease.
The ubiquitin proteasomal system degrades nuclear cGAS in cycling cells.
SPSB3 is identified as the cGAS-targeting substrate receptor associated with the CRL5 complex.
A cryo-electron microscopy structure reveals a conserved degron motif at the C terminus of cGAS directing SPSB3 recruitment.
Interference with SPSB3-regulated nuclear cGAS degradation enhances protection against infection by DNA viruses.
引述
"Our research defines protein degradation as a determinant of cGAS regulation in the nucleus."
"The indiscriminate activity of cGAS towards DNA demands tight regulatory mechanisms."
從以下內容提煉的關鍵洞見
by Pengbiao Xu,... 於 www.nature.com 02-28-2024
https://www.nature.com/articles/s41586-024-07112-w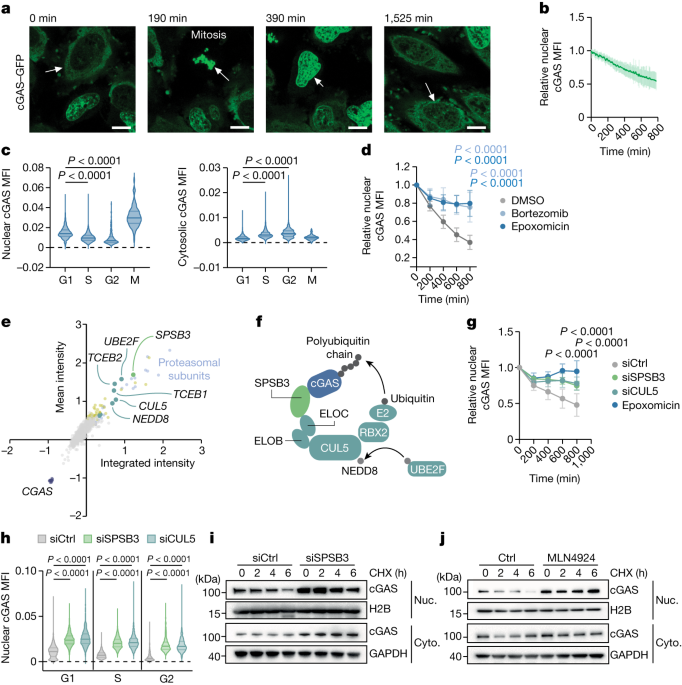
深入探究
How can targeting nuclear cGAS degradation impact therapeutic strategies for infectious diseases?
Targeting nuclear cGAS degradation can have significant implications for therapeutic strategies in infectious diseases. By understanding the role of the CRL5–SPSB3 ubiquitin ligase complex in regulating the degradation of nuclear cGAS, researchers can potentially develop targeted therapies that modulate this pathway to enhance immune responses against pathogens. Inhibiting SPSB3-mediated degradation of cGAS could lead to increased activation of innate immune signaling pathways, particularly type I interferon responses, which play a crucial role in antiviral defense mechanisms. This approach may help boost the host's ability to combat viral infections by promoting a more robust immune response against DNA viruses.
What are potential implications of interfering with SPSB3-regulated degradation on autoimmune conditions?
Interfering with SPSB3-regulated degradation and subsequently stabilizing nuclear cGAS could have complex implications for autoimmune conditions. While enhancing cGAS stability may promote stronger innate immune responses against pathogens, it also raises concerns about potential hyperactivation of inflammatory pathways that could contribute to autoimmunity. Dysregulation of nucleic acid sensing pathways, including those involving cGAS, has been implicated in various autoimmune disorders where self-DNA or RNA triggers aberrant immune reactions. Therefore, disrupting SPSB3-mediated degradation as a therapeutic strategy would need careful consideration and fine-tuning to avoid exacerbating autoimmune conditions by inadvertently triggering excessive inflammation and tissue damage.
How might understanding protein degradation mechanisms lead to novel approaches in cancer treatment?
Understanding protein degradation mechanisms, such as the UPS-mediated regulation of nuclear cGAS through SPSB3 targeting, opens up new avenues for developing innovative approaches in cancer treatment. Targeted manipulation of protein turnover processes within cancer cells can be leveraged to selectively degrade oncogenic proteins or disrupt key signaling pathways involved in tumorigenesis. In the context of cGAS regulation, modulating its stability via interference with SPSB3-mediated ubiquitination could potentially influence anti-tumor immunity by enhancing immunosurveillance against malignant cells.
By elucidating how specific proteins are targeted for degradation and identifying critical regulatory components like E3 ligases and substrate receptors involved in these processes, researchers can design tailored therapeutics that exploit protein turnover dynamics to inhibit tumor growth or sensitize cancer cells to existing treatments like chemotherapy or immunotherapy. This knowledge paves the way for precision medicine strategies that target dysregulated protein homeostasis mechanisms unique to cancer cells while sparing normal tissues from collateral damage during treatment interventions.
0
