Cellular Plasticity and Architectural Remodeling in Human Chronic Liver Disease
Core Concepts
Chronic liver injury induces cellular plasticity and architectural remodeling in the human liver, involving transdifferentiation between hepatocytes and cholangiocytes without the presence of adult stem cells or developmental progenitor activation.
Abstract
The article investigates the regenerative processes in the human liver during chronic disease, which remain a controversial subject compared to well-characterized animal models. The researchers performed single-nucleus RNA sequencing (snRNA-seq) on 47 liver biopsies from patients with different stages of metabolic dysfunction-associated steatotic liver disease to establish a cellular map of the liver during disease progression. They combined these single-cell-level data with advanced 3D imaging to reveal profound changes in the liver architecture.
The key findings include:
Hepatocytes lose their zonation, and considerable reorganization of the biliary tree takes place.
The study uncovers transdifferentiation events between hepatocytes and cholangiocytes without the presence of adult stem cells or developmental progenitor activation.
Detailed analyses and functional validations using cholangiocyte organoids confirm the importance of the PI3K–AKT–mTOR pathway in this process, connecting the acquisition of plasticity to insulin signaling.
The data indicates that chronic injury creates an environment that induces cellular plasticity in the human liver, and understanding the underlying mechanisms could open new therapeutic avenues in the management of chronic diseases.
Acquisition of epithelial plasticity in human chronic liver disease - Nature
Stats
We performed single-nucleus RNA sequencing (snRNA-seq) on 47 liver biopsies from patients with different stages of metabolic dysfunction-associated steatotic liver disease.
Detailed analyses and functional validations using cholangiocyte organoids confirm the importance of the PI3K–AKT–mTOR pathway in the acquisition of cellular plasticity.
Quotes
"Together, our data indicate that chronic injury creates an environment that induces cellular plasticity in human organs, and understanding the underlying mechanisms of this process could open new therapeutic avenues in the management of chronic diseases."
Key Insights Distilled From
by Christopher ... at www.nature.com 05-22-2024
https://www.nature.com/articles/s41586-024-07465-2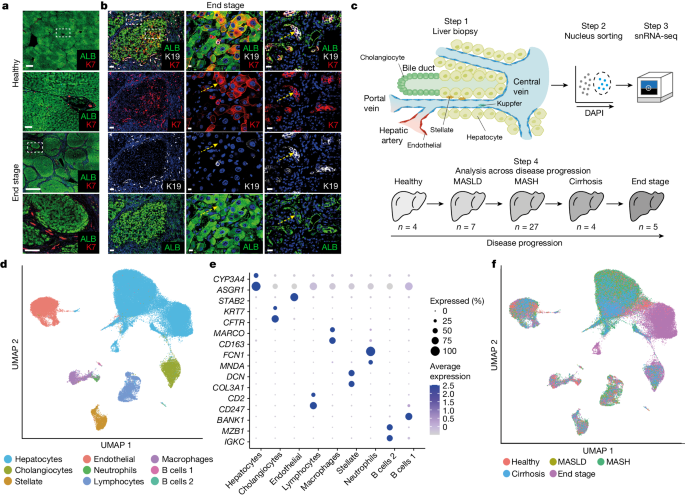
Deeper Inquiries
What are the potential implications of the observed cellular plasticity in the human liver for the development of new therapeutic strategies for chronic liver diseases?
The observed cellular plasticity in the human liver, where hepatocytes and cholangiocytes undergo transdifferentiation without the involvement of adult stem cells or developmental progenitors, opens up new avenues for therapeutic strategies in chronic liver diseases. Understanding the underlying mechanisms, such as the role of the PI3K–AKT–mTOR pathway and its connection to insulin signaling, provides a potential target for intervention. By targeting these pathways, it may be possible to manipulate cellular plasticity in the liver, leading to the development of novel treatments that can promote regeneration and repair in the context of chronic liver diseases.
How do the findings in this study compare to the regenerative processes observed in animal models of liver injury, and what are the key differences?
The findings in this study shed light on the regenerative processes in the human liver during chronic disease, which have been challenging to study due to technical and ethical constraints. While regenerative processes in animal models have been well characterized and often involve the activation of adult stem cells or progenitors, the observed transdifferentiation events between hepatocytes and cholangiocytes in human chronic liver disease represent a unique mechanism not typically seen in animal models. This highlights a key difference in the regenerative processes between humans and animals, emphasizing the importance of studying human tissues to understand disease progression and potential therapeutic targets accurately.
Could the mechanisms underlying the acquisition of cellular plasticity in the liver be applicable to other human organs affected by chronic disease, and if so, what would be the broader implications?
The mechanisms underlying the acquisition of cellular plasticity in the liver, particularly the involvement of the PI3K–AKT–mTOR pathway and insulin signaling, could have broader implications for other human organs affected by chronic disease. If similar transdifferentiation events can be induced in other tissues, it may pave the way for novel therapeutic strategies in various chronic diseases. Understanding how chronic injury creates an environment that induces cellular plasticity could lead to the development of targeted interventions that promote regeneration and repair in a wide range of organs, potentially revolutionizing the treatment of chronic diseases beyond the liver.
0
