Direct Observation of Atomic-Scale Dynamics at Electrified Solid-Liquid Interfaces during Electrochemical Reactions
Core Concepts
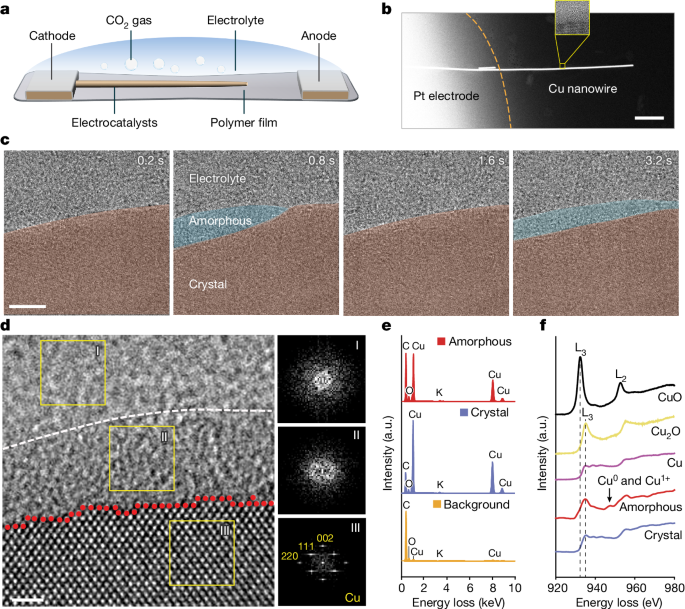
Electrified solid-liquid interfaces undergo reversible crystalline-amorphous structural transformations and flow along the surface, mediating surface restructuring and mass loss during electrochemical reactions.
Abstract
The content describes the direct observation of atomic-scale dynamics at electrified solid-liquid interfaces (ESLIs) during electrochemical reactions, enabled by the development of advanced polymer electrochemical liquid cells for transmission electron microscopy (TEM).
The key highlights and insights are:
-
ESLIs play a crucial role in various electrochemical processes relevant to energy, biology, and geochemistry, as the electron and mass transport at these interfaces can result in structural modifications that influence reaction pathways.
-
Directly probing the atomic dynamics of solid-liquid interfaces under electric biasing is challenging due to the buried nature of the interfaces in liquid electrolytes and the limited spatial resolution of current in situ imaging techniques.
-
The researchers used their developed polymer electrochemical liquid cells for TEM to directly monitor the atomic dynamics of ESLIs during copper-catalyzed CO2 electroreduction reactions.
-
The observation reveals a fluctuating liquid-like amorphous interphase at the ESLI, which undergoes reversible crystalline-amorphous structural transformations and flows along the electrified copper surface.
-
This amorphous interphase mediates the crystalline copper surface restructuring and mass loss through the interphase layer.
-
The combination of real-time observation and theoretical calculations unveils an amorphization-mediated restructuring mechanism resulting from charge-activated surface reactions with the electrolyte.
-
The results open up opportunities to explore the atomic dynamics and its impact in a broad range of systems involving ESLIs, leveraging the in situ imaging capability.
Translate Source
To Another Language
Generate MindMap
from source content
Visit Source
www.nature.com
Atomic dynamics of electrified solid–liquid interfaces in liquid-cell TEM - Nature
Stats
Electrified solid-liquid interfaces play a key role in various electrochemical processes relevant to energy, biology and geochemistry.
Directly probing the atomic dynamics of solid-liquid interfaces under electric biasing is challenging due to the buried nature of the interfaces in liquid electrolytes and the limited spatial resolution of current in situ imaging techniques.
Quotes
"Our observation reveals a fluctuating liquid-like amorphous interphase. It undergoes reversible crystalline–amorphous structural transformations and flows along the electrified Cu surface, thus mediating the crystalline Cu surface restructuring and mass loss through the interphase layer."
"The combination of real-time observation and theoretical calculations unveils an amorphization-mediated restructuring mechanism resulting from charge-activated surface reactions with the electrolyte."
Deeper Inquiries
How can the insights from this study be leveraged to design more efficient and stable electrocatalysts for energy conversion and storage applications?
The insights gained from this study on the atomic dynamics of electrified solid-liquid interfaces (ESLIs) can be instrumental in designing electrocatalysts with improved efficiency and stability for energy conversion and storage applications. By understanding the mechanisms of amorphization-mediated restructuring observed in the copper-based system during CO2 electroreduction reactions, researchers can tailor the composition and structure of electrocatalysts to promote similar dynamic behaviors that enhance catalytic activity. For instance, incorporating elements or functional groups that facilitate reversible structural transformations at the solid-liquid interface could lead to more active and durable electrocatalysts. Additionally, strategies to control the flow and properties of the liquid-like amorphous interphase could be employed to optimize mass transport and reaction kinetics, ultimately improving the overall performance of electrocatalytic systems for energy applications.
What other types of solid-liquid interfaces, beyond the copper-based system studied here, could exhibit similar amorphization-mediated restructuring dynamics, and how might this impact their functionality?
Beyond copper, various other solid-liquid interfaces could exhibit similar amorphization-mediated restructuring dynamics, especially in systems involving electrochemical reactions or interactions with liquid electrolytes. For example, noble metals like platinum and silver, commonly used in electrocatalysis, may undergo comparable structural transformations at the solid-liquid interface under electric biasing. Transition metal oxides, such as cobalt oxide or manganese oxide, which are also important for energy conversion reactions, could display similar amorphization-mediated restructuring dynamics during electrochemical processes. The impact of such dynamics on the functionality of these materials could be significant, influencing their catalytic activity, selectivity, and stability. Understanding and controlling these restructuring phenomena could enable the design of more efficient and durable electrocatalysts for a wide range of applications.
Given the importance of understanding atomic-scale dynamics at electrified interfaces, what advancements in in situ imaging and characterization techniques would be most impactful for further exploring these phenomena?
To further explore the atomic-scale dynamics at electrified interfaces, advancements in in situ imaging and characterization techniques are crucial. One impactful advancement would be the development of high-resolution liquid-cell transmission electron microscopy (TEM) techniques that allow for real-time monitoring of ESLIs with improved spatial and temporal resolution. This would enable direct observation of atomic rearrangements and structural transformations at the solid-liquid interface under electric biasing, providing detailed insights into the dynamic processes occurring during electrochemical reactions. Additionally, the integration of spectroscopic techniques, such as in situ X-ray spectroscopy or vibrational spectroscopy, with imaging methods could offer complementary information on the chemical and electronic changes accompanying the structural dynamics at electrified interfaces. Furthermore, advancements in computational modeling and simulation approaches could help interpret experimental observations and predict the behavior of ESLIs under different conditions, facilitating the rational design of electrocatalysts with tailored properties and enhanced performance.