Discovery of a DNA Glycosylase Enzyme that Provides Antiviral Defense in Bacteria Against Bacteriophage T4 Infection
The content describes the discovery of a novel bacterial defense system against bacteriophage infection. Researchers infected Escherichia coli carrying a soil metagenomic DNA library with the lytic coliphage T4 to isolate clones carrying protective genes. Through this approach, they identified Brig1, a DNA glycosylase enzyme that can excise α-glucosyl-hydroxymethylcytosine nucleobases from the T4 phage genome, generating abasic sites and inhibiting viral replication.
The authors found that Brig1 homologues providing immunity against T-even phages are present in multiple phage defense loci across distinct clades of bacteria. This highlights the importance of DNA glycosylases as key players in the ongoing bacteria-phage arms race. The study demonstrates the benefits of screening unsequenced DNA to discover novel defense systems, as the available prokaryotic sequence data may not capture the full diversity of antiviral mechanisms evolved by bacteria.
Customize Summary
Rewrite with AI
Generate Citations
Translate Source
To Another Language
Generate MindMap
from source content
Visit Source
www.nature.com
DNA glycosylases provide antiviral defence in prokaryotes - Nature
Key Insights Distilled From
by Amer A. Hoss... at www.nature.com 04-17-2024
https://www.nature.com/articles/s41586-024-07329-9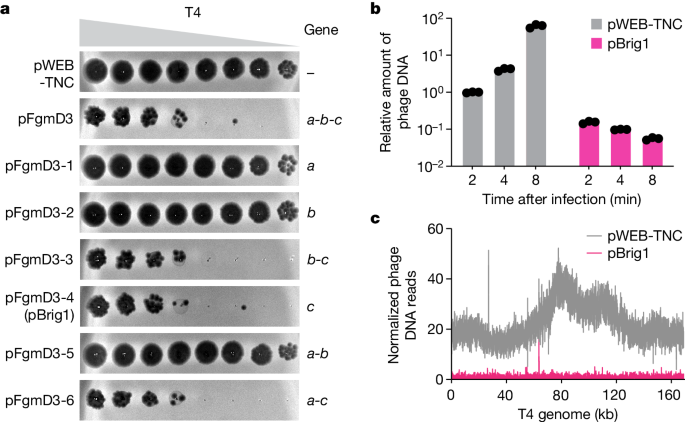
Deeper Inquiries
