insight - Computational Biology - # Structural Insights into Anti-Tuberculosis Drugs Targeting ATP Synthase
High-Resolution Structural Analysis of Anti-Tuberculosis Drugs Targeting ATP Synthase in Mycobacterium tuberculosis and Humans
Core Concepts
Structural analysis of how anti-tuberculosis drugs bedaquiline and TBAJ-587 interact with ATP synthases from Mycobacterium tuberculosis and humans, providing crucial insights for developing safer and more effective tuberculosis treatments.
Abstract
The content discusses the discovery of a class of compounds in 2005 that could inhibit the bacterium Mycobacterium tuberculosis, which causes tuberculosis infections in humans. Unexpectedly, the target of these drugs was ATP synthase, the enzyme responsible for producing cellular energy in the form of ATP molecules. This raised concerns that antimicrobials targeting this enzyme would also target the human version, causing toxic side effects.
The article then describes the work by Zhang et al., who provide the first high-resolution structural insights into how the anti-tuberculosis drugs bedaquiline and its derivative TBAJ-587 interact with the ATP synthases from M. tuberculosis and humans. This structural analysis is crucial for developing safer and more effective tuberculosis treatments, as it helps understand the differences between the bacterial and human versions of the ATP synthase enzyme, which can be exploited to design drugs that selectively target the bacterial enzyme without affecting the human version.
Blueprints for ATP machinery will aid tuberculosis drug design
Stats
In 2005, a class of compound was discovered that could inhibit the bacterium Mycobacterium tuberculosis.
The target of these drugs was ATP synthase, the enzyme responsible for producing cellular energy in the form of ATP molecules.
The evolutionary conservation of ATP synthase enzymes across all domains of life had prompted concerns that antimicrobials targeting this enzyme would also target the human version, causing toxic side effects in humans.
Quotes
"Writing in Nature, Zhang et al.2 provide the first high-resolution structural insights into how these anti-tuberculosis drugs — namely bedaquiline and its derivative TBAJ-587 — interact with the ATP synthases from M. tuberculosis and, crucially, humans."
Key Insights Distilled From
by Gregory M. C... at www.nature.com 07-03-2024
https://www.nature.com/articles/d41586-024-02094-1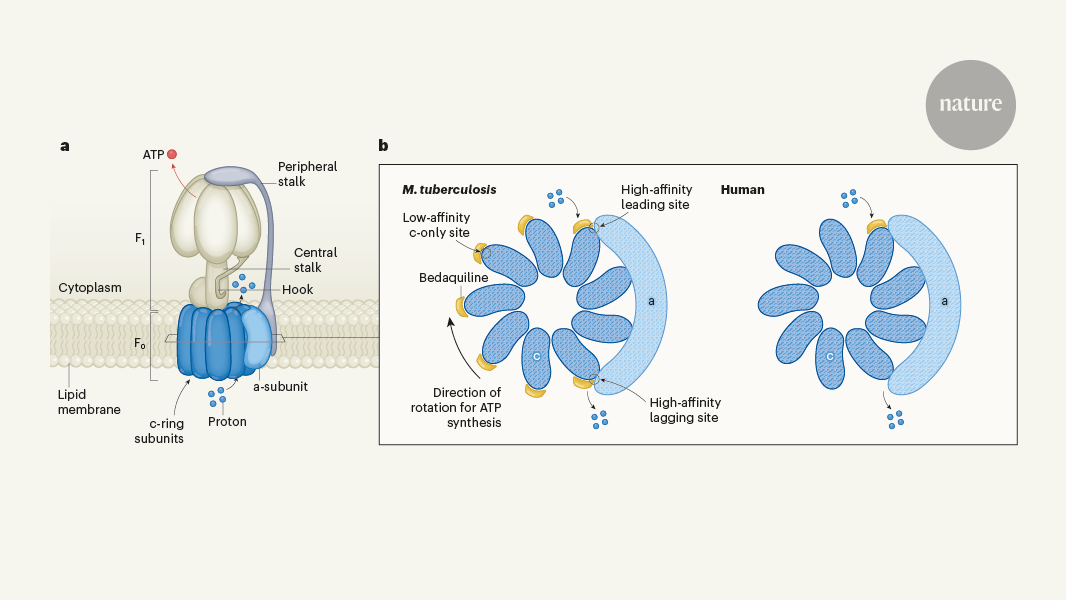
Deeper Inquiries
What are the key structural differences between the ATP synthase enzymes of Mycobacterium tuberculosis and humans that enable the selective targeting of the bacterial enzyme by the anti-tuberculosis drugs?
The key structural differences between the ATP synthase enzymes of Mycobacterium tuberculosis and humans lie in specific regions of the enzyme that are targeted by the anti-tuberculosis drugs. In the case of bedaquiline and its derivative TBAJ-587, these compounds interact with unique binding sites on the ATP synthase of M. tuberculosis that are not present or significantly differ in the human version of the enzyme. This selective targeting is crucial for the efficacy of the drugs against the bacterial enzyme while minimizing the risk of toxic side effects in humans. By understanding the structural differences in these binding sites, researchers can design drugs that specifically target the bacterial ATP synthase without affecting the human counterpart.
How can the structural insights provided in this study be leveraged to design even more selective and potent anti-tuberculosis drugs that minimize the risk of toxic side effects in humans?
The structural insights from this study offer a detailed understanding of how anti-tuberculosis drugs interact with the ATP synthase enzymes of both Mycobacterium tuberculosis and humans. By leveraging this knowledge, researchers can utilize structure-based drug design approaches to develop even more selective and potent anti-tuberculosis drugs. This can involve modifying the chemical structure of existing drugs to enhance their specificity for the bacterial enzyme while reducing their affinity for the human enzyme. Additionally, computational modeling and virtual screening techniques can be employed to identify novel compounds that target unique regions of the bacterial ATP synthase, further minimizing the risk of toxic side effects in humans.
Given the evolutionary conservation of ATP synthase enzymes, what other potential applications or therapeutic targets could this structural analysis inform beyond the treatment of tuberculosis?
The structural analysis of ATP synthase enzymes can have implications beyond the treatment of tuberculosis in various therapeutic areas. One potential application is in the development of antimicrobial agents against other bacterial pathogens that rely on ATP synthase for energy production. By understanding the structural features that differentiate bacterial ATP synthases from their human counterparts, researchers can design selective inhibitors for specific bacterial species while sparing human cells. Moreover, this structural analysis can inform drug discovery efforts in cancer research, as ATP synthase is also implicated in tumor metabolism. Targeting cancer-specific ATP synthase isoforms could lead to the development of novel anticancer therapies with improved efficacy and reduced off-target effects.
0
