Innovative Mitochondrial Transplantation Strategy to Repair Ischemic Tissue Damage
Core Concepts
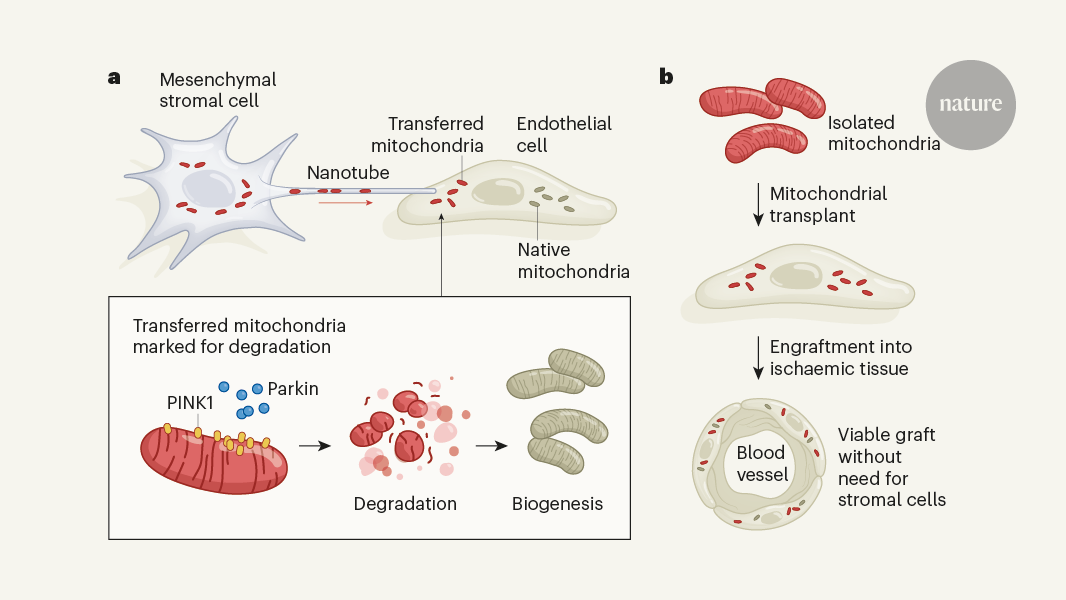
Transplanting mitochondria from one cell to another can aid the repair of ischemic tissue damage by enhancing energy production and waste clearance in affected areas.
Abstract
The article discusses an innovative transplantation strategy that involves the transfer of energy-generating organelles called mitochondria from one cell to another to aid the repair of ischemic tissue. Ischemia, a condition where blood supply and waste clearance are prevented, can lead to various cardiovascular diseases such as coronary heart disease and heart attack.
The key highlights of the article are:
-
Blood vessels play a crucial role in delivering oxygen and nutrients to tissues while removing waste products. When these vessels become narrow or blocked, it results in ischemia, which can cause serious health conditions.
-
The authors report a novel transplantation approach that utilizes the transfer of mitochondria, the energy-generating organelles, from one cell to another to help repair ischemic tissue damage.
-
This strategy aims to enhance energy production and waste clearance in the affected areas, thereby improving the overall function and recovery of the ischemic tissue.
-
The article suggests that this innovative mitochondrial transplantation technique could potentially be a valuable tool in the treatment and management of various ischemic-related diseases, such as coronary heart disease and heart attack.
Translate Source
To Another Language
Generate MindMap
from source content
Visit Source
www.nature.com
Cells destroy donated mitochondria to build blood vessels
Stats
Blood vessels deliver oxygen and nutrients to tissues and remove waste products from them.
When blood vessels become narrow or blocked, it results in ischemia, which can lead to conditions such as coronary heart disease and heart attack.
Quotes
"When these vessels become narrow or blocked, the blood supply and waste clearance are prevented, resulting in what is called ischaemia, which in turn leads to conditions such as coronary heart disease and heart attack."
Deeper Inquiries
How can the efficiency and scalability of the mitochondrial transplantation technique be improved to make it a more viable clinical solution?
To enhance the efficiency and scalability of the mitochondrial transplantation technique for clinical applications, several strategies can be implemented. Firstly, optimizing the isolation and purification methods for extracting functional mitochondria from donor cells can improve the quality of the transferred organelles. This can involve developing standardized protocols that ensure high mitochondrial integrity and functionality post-transplantation. Additionally, exploring alternative delivery methods, such as using nanocarriers or exosomes to transport mitochondria to target tissues, can enhance the specificity and efficacy of the transplantation process. Moreover, conducting further research to understand the mechanisms underlying mitochondrial transfer between cells and the factors influencing their uptake and integration can help streamline the transplantation procedure and maximize its therapeutic benefits. Collaborative efforts between researchers, clinicians, and biotechnologists are essential to refine the mitochondrial transplantation technique and make it a more practical and scalable solution for clinical use.
What potential challenges or limitations might arise in the practical implementation of this mitochondrial transplantation strategy, and how could they be addressed?
Despite the promising potential of mitochondrial transplantation for treating ischemic conditions, several challenges and limitations may arise during its practical implementation. One major concern is the immune response triggered by the introduction of exogenous mitochondria into the recipient's cells, which could lead to rejection or adverse reactions. To address this issue, researchers can explore methods to modify the surface properties of donor mitochondria to evade immune detection or develop immunomodulatory strategies to promote tolerance towards the transplanted organelles. Another challenge is ensuring the long-term stability and functionality of the transferred mitochondria within the host cells, as factors like mitochondrial turnover and quality control mechanisms may affect their persistence and performance. By investigating ways to enhance the integration and maintenance of donor mitochondria in the recipient cells, such as promoting fusion with endogenous mitochondria or modulating mitochondrial dynamics, researchers can overcome this limitation and improve the overall success rate of the transplantation strategy.
Given the role of mitochondria in cellular energy production, how might this technology be leveraged to address other types of tissue damage or metabolic disorders beyond ischemic conditions?
The technology of mitochondrial transplantation holds great potential for addressing a wide range of tissue damage and metabolic disorders beyond ischemic conditions, owing to the central role of mitochondria in cellular energy production and homeostasis. For instance, in neurodegenerative diseases like Parkinson's or Alzheimer's, where mitochondrial dysfunction plays a critical role in disease progression, targeted delivery of healthy mitochondria to affected neurons could help restore cellular bioenergetics and mitigate neuronal damage. Similarly, in metabolic disorders such as diabetes or obesity, where impaired mitochondrial function contributes to metabolic dysregulation, transplanting functional mitochondria to tissues like adipose or muscle cells could enhance energy metabolism and improve overall metabolic health. By tailoring the mitochondrial transplantation approach to specific tissue types and disease contexts, researchers can harness the therapeutic potential of this technology to combat a diverse array of pathologies associated with mitochondrial dysfunction and energy deficits.