insight - Computational Biology - # Molecular Characterization of Toll-like Receptor Signaling Pathways
Molecular Mechanisms Underlying Endogenous Toll-like Receptor Signaling Pathways
Core Concepts
The entire Toll-like receptor signaling pathway is executed from within the myddosome, a dynamic scaffold that coordinates the recruitment and activation of effector proteins.
Abstract
This article provides a detailed molecular characterization of the endogenous Toll-like receptor (TLR) signaling pathways. The key findings are:
TLR activation leads to the formation of transient contacts between the receptors and the myddosome, a protein complex containing the adaptor protein MyD88.
TLR-free myddosomes are dynamic in size, number, and composition over 24 hours, suggesting they play a broader role in regulating immune responses.
Super-resolution microscopy revealed that MyD88 forms barrel-like structures within myddosomes, which serve as scaffolds for recruiting effector proteins.
Proteomic analysis showed that myddosomes contain proteins involved in all stages and effector responses of the TLR pathways, and genetic analysis defined the epistatic relationships between these effector modules.
Myddosome assembly was observed in cells infected with Listeria monocytogenes, but the bacteria were able to evade myddosome-mediated TLR signaling during cell-to-cell spread.
Based on these findings, the authors propose that the entire TLR signaling pathway is executed from within the myddosome, a dynamic molecular platform that coordinates the detection of pathogens and the activation of downstream immune responses.
Molecular definition of the endogenous Toll-like receptor signalling pathways - Nature
Stats
Toll-like receptors (TLRs) are key mediators of the immune response to infection.
Myddosomes form transient contacts with activated TLRs.
TLR-free myddosomes are dynamic in size, number, and composition over 24 hours.
MyD88 forms barrel-like structures within myddosomes that function as scaffolds for effector protein recruitment.
Myddosomes contain proteins involved in all stages and effector responses of the TLR pathways.
Listeria monocytogenes can evade myddosome-mediated TLR signaling during cell-to-cell spread.
Quotes
"We found that myddosomes form transient contacts with activated TLRs and that TLR-free myddosomes are dynamic in size, number and composition over the course of 24 h."
"Analysis using super-resolution microscopy revealed that, within most myddosomes, MyD88 forms barrel-like structures that function as scaffolds for effector protein recruitment."
"Proteomic analysis demonstrated that myddosomes contain proteins that act at all stages and regulate all effector responses of the TLR pathways, and genetic analysis defined the epistatic relationship between these effector modules."
Key Insights Distilled From
by Daniel Fisch... at www.nature.com 07-03-2024
https://www.nature.com/articles/s41586-024-07614-7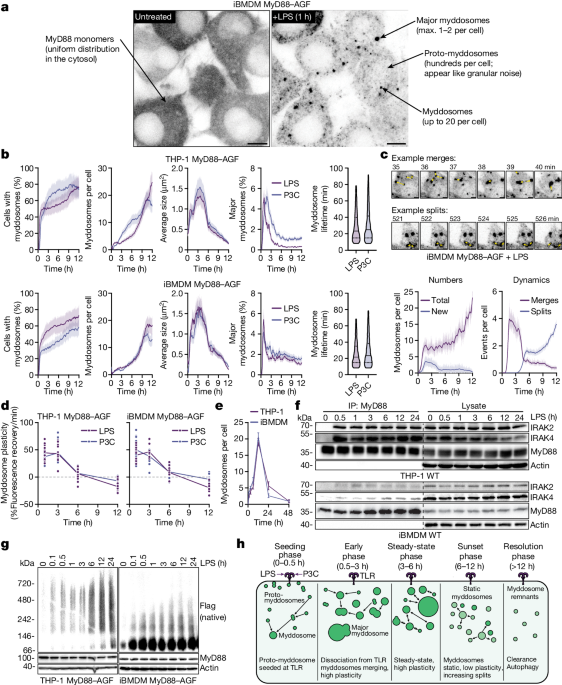
Deeper Inquiries
How do the dynamic properties of myddosomes contribute to the regulation of broader immune responses beyond TLR signaling?
The dynamic properties of myddosomes play a crucial role in regulating broader immune responses beyond TLR signaling by facilitating the assembly of effector proteins and coordinating downstream signaling events. As mentioned in the context, myddosomes form transient contacts with activated TLRs, allowing for the recruitment of various effector proteins that are essential for initiating and propagating immune responses. The dynamic nature of myddosomes in terms of size, number, and composition over time enables the fine-tuning of immune responses based on the specific pathogen encountered. This adaptability ensures that the immune system can mount an appropriate and effective response to different types of infections, thereby contributing to the regulation of broader immune responses beyond TLR signaling.
What are the specific mechanisms by which Listeria monocytogenes evades myddosome-mediated TLR signaling during cell-to-cell spread?
Listeria monocytogenes has evolved specific mechanisms to evade myddosome-mediated TLR signaling during cell-to-cell spread, allowing it to subvert the host immune response and facilitate its survival and dissemination. One key mechanism employed by Listeria monocytogenes is the evasion of myddosome assembly, thereby preventing the activation of downstream TLR signaling pathways. By evading myddosome formation, Listeria monocytogenes can avoid the recruitment of effector proteins necessary for mounting an effective immune response against the pathogen. Additionally, Listeria monocytogenes may possess virulence factors or proteins that interfere with the interaction between TLRs and myddosomes, disrupting the signaling cascade and dampening the host immune response. These evasion strategies enable Listeria monocytogenes to establish a replicative niche within host cells and promote its spread from cell to cell without eliciting a robust immune reaction mediated by myddosomes and TLR signaling.
Could the insights into myddosome structure and function be leveraged to develop new therapeutic strategies for modulating innate immune responses in the context of infection or inflammation?
The insights gained from studying myddosome structure and function hold significant promise for the development of novel therapeutic strategies aimed at modulating innate immune responses in the context of infection or inflammation. By understanding the intricate mechanisms by which myddosomes orchestrate TLR signaling and immune responses, researchers can identify potential targets for therapeutic intervention to enhance or suppress immune reactions as needed. For instance, targeting specific components of the myddosome complex or modulating the assembly of myddosomes could offer new avenues for fine-tuning immune responses to combat infections or mitigate inflammatory conditions. Furthermore, insights into myddosome dynamics and protein interactions could inform the design of small molecules or biologics that selectively modulate TLR signaling pathways, providing more precise and effective therapeutic options for managing immune-related disorders. Leveraging the knowledge of myddosome structure and function may thus lead to the development of innovative therapies that harness the innate immune system's capabilities to combat pathogens or alleviate inflammatory responses in a targeted manner.
0
