Multiciliated Cell Differentiation Coordinated by an Alternative Cell Cycle Distinct from the Canonical Mitotic Cell Cycle
Core Concepts
Multiciliated cells use an alternative cell cycle, the "multiciliation cycle", which redeploys canonical cell cycle regulators to coordinate centriole amplification and ciliogenesis instead of DNA replication and cell division.
Abstract
The content describes how differentiating multiciliated cells, found in the mammalian airway, brain ventricles, and reproductive tract, use an alternative cell cycle distinct from the canonical mitotic cell cycle. This alternative cell cycle, referred to as the "multiciliation cycle", coordinates the generation of hundreds of centrioles and their maturation into basal bodies that nucleate motile cilia.
The multiciliation cycle redeploys many canonical cell cycle regulators, such as cyclin-dependent kinases (CDKs) and their cognate cyclins. For example, cyclin D1, CDK4, and CDK6, which regulate mitotic G1-to-S progression, are required to initiate multiciliated cell differentiation. The multiciliation cycle amplifies certain aspects of the canonical cell cycle, like centriole synthesis, while blocking others, such as DNA replication.
The transcriptional regulator E2F7, which normally controls canonical S-to-G2 progression, is expressed at high levels during the multiciliation cycle. E2F7 directly dampens the expression of genes encoding DNA replication machinery and terminates the S phase-like gene expression program, preventing DNA synthesis in multiciliated cells. Loss of E2F7 leads to aberrant DNA synthesis and dysregulation of the multiciliation cycle, disrupting centriole maturation and ciliogenesis.
The content concludes that multiciliated cells use this alternative cell cycle to orchestrate their differentiation, rather than controlling proliferation like the canonical mitotic cell cycle.
An alternative cell cycle coordinates multiciliated cell differentiation - Nature
Stats
Multiciliated cells generate hundreds of centrioles, each of which matures into a basal body and nucleates a motile cilium.
Loss of E2F7 causes aberrant DNA synthesis in multiciliated cells and disrupts centriole maturation and ciliogenesis.
Quotes
"The multiciliation cycle redeploys many canonical cell cycle regulators, including cyclin-dependent kinases (CDKs) and their cognate cyclins."
"E2F7 directly dampens the expression of genes encoding DNA replication machinery and terminates the S phase-like gene expression program, preventing DNA synthesis in multiciliated cells."
Key Insights Distilled From
by Semil P. Cho... at www.nature.com 05-29-2024
https://www.nature.com/articles/s41586-024-07476-z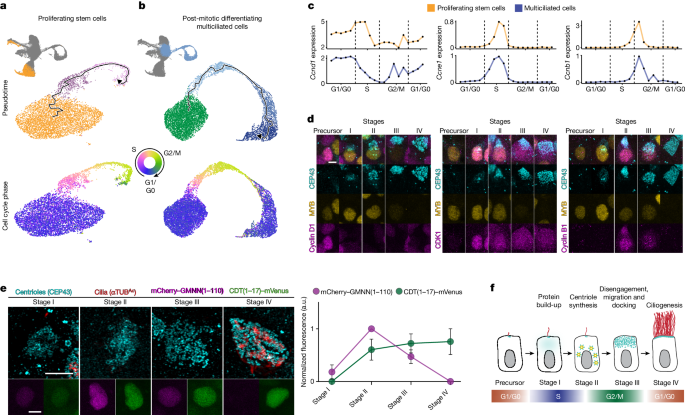
Deeper Inquiries
How do the specific mechanisms and regulatory networks of the multiciliation cycle differ from the canonical mitotic cell cycle?
In the multiciliation cycle, there is a distinct reorganization of cell cycle regulators compared to the canonical mitotic cell cycle. While the canonical mitotic cell cycle coordinates processes like DNA replication, centriole duplication, and cytokinesis to produce two daughter cells, the multiciliation cycle is a specialized cell cycle that drives the differentiation of multiciliated cells. This alternative cell cycle involves the redeployment of key regulators such as cyclin-dependent kinases (CDKs) and their cognate cyclins. For instance, cyclin D1, CDK4, and CDK6, which typically regulate G1-to-S progression in the canonical cell cycle, play essential roles in initiating multiciliated cell differentiation. Additionally, the multiciliation cycle amplifies centriole synthesis while blocking DNA replication, indicating a divergence from the canonical cell cycle mechanisms. Furthermore, transcriptional regulators like E2F7 are highly expressed during the multiciliation cycle, where they suppress genes involved in DNA replication and promote the differentiation program, showcasing a unique regulatory network specific to multiciliated cell differentiation.
What are the potential implications of the multiciliation cycle for understanding cell differentiation and tissue development beyond multiciliated cells?
Studying the multiciliation cycle not only provides insights into the specialized differentiation process of multiciliated cells but also offers valuable knowledge for understanding broader aspects of cell differentiation and tissue development. By unraveling the regulatory mechanisms and signaling pathways involved in the multiciliation cycle, researchers can gain a deeper understanding of how cells transition from a proliferative state to a differentiated, post-mitotic state. This knowledge can be extrapolated to other cell types and tissues undergoing differentiation, shedding light on the intricate molecular events that drive cellular specialization and tissue morphogenesis. Understanding the multiciliation cycle may uncover common principles and regulatory networks that govern cell fate decisions and developmental processes across various cell types, thereby advancing our comprehension of tissue development and regeneration beyond multiciliated cells.
Could the insights into the multiciliation cycle be leveraged to engineer or manipulate multiciliated cell differentiation for therapeutic or bioengineering applications?
The detailed understanding of the multiciliation cycle and its regulatory mechanisms opens up possibilities for leveraging this knowledge in therapeutic and bioengineering applications. By deciphering the key factors and pathways that drive multiciliated cell differentiation, researchers can potentially engineer strategies to enhance or manipulate this process for therapeutic purposes. For instance, targeting specific regulators or signaling molecules identified in the multiciliation cycle could be a promising approach to modulate multiciliated cell differentiation in conditions where cilia function is impaired or needs to be augmented. Moreover, the insights gained from studying the multiciliation cycle could be applied in bioengineering contexts to develop novel approaches for generating multiciliated cells in vitro for various applications, such as modeling cilia-related disorders or creating functional multiciliated cell systems for drug screening and regenerative medicine purposes. Leveraging the knowledge of the multiciliation cycle may thus offer new avenues for therapeutic interventions and bioengineering advancements related to multiciliated cells.
0
