insight - Computational Biology - # Intergenerational Effects of Paternal Gut Microbiome Perturbations
Paternal Gut Microbiome Disruptions Negatively Impact Offspring Health and Survival
Core Concepts
Perturbations to the gut microbiome of prospective fathers can increase the risk of their offspring experiencing low birth weight, growth restriction, and premature mortality.
Abstract
This study investigates the systemic impact of the gut microbiome on the germline and subsequent effects on offspring fitness. The researchers found that disruptions to the gut microbiota of male mice, induced by non-absorbable antibiotics or osmotic laxatives, increased the probability of their offspring presenting with low birth weight, severe growth restriction, and premature mortality.
The transmission of this disease risk occurs via the germline and is linked to a dynamic response in the male reproductive system, including impaired leptin signaling, altered testicular metabolite profiles, and remapped small RNA payloads in sperm. These paternal microbiome perturbations trigger an elevated risk of in utero placental insufficiency, revealing a placental origin of the observed intergenerational effects.
The study defines a "gut-germline axis" in males that is sensitive to environmental exposures and can program offspring fitness through impacts on placental function. Restoring the paternal microbiota before conception was able to rescue the adverse effects on offspring, highlighting the importance of a healthy gut microbiome for intergenerational health.
Paternal microbiome perturbations impact offspring fitness - Nature
Stats
Perturbations to the gut microbiome of prospective fathers increased the probability of their offspring presenting with low birth weight, severe growth restriction, and premature mortality.
Restoring the paternal microbiota before conception rescued the adverse effects on offspring.
Quotes
"Perturbations to the gut microbiota of prospective fathers increase the probability of their offspring presenting with low birth weight, severe growth restriction and premature mortality."
"This effect is linked with a dynamic response to induced dysbiosis in the male reproductive system, including impaired leptin signalling, altered testicular metabolite profiles and remapped small RNA payloads in sperm."
Key Insights Distilled From
by Ayele Argaw-... at www.nature.com 05-01-2024
https://www.nature.com/articles/s41586-024-07336-w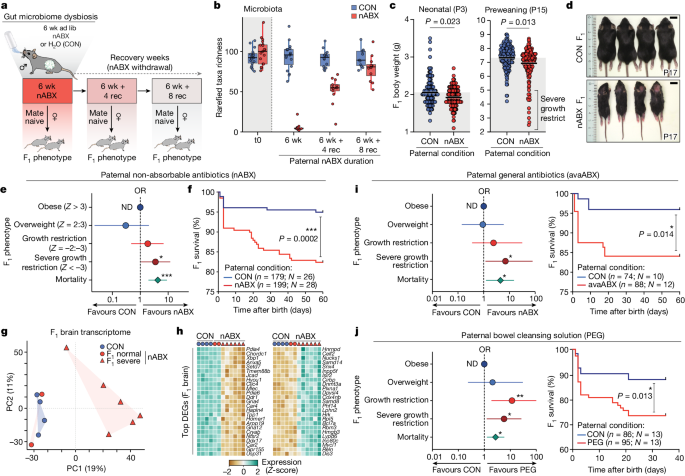
Deeper Inquiries
What are the specific mechanisms by which paternal gut microbiome perturbations impact placental function and lead to adverse offspring outcomes?
The impact of paternal gut microbiome perturbations on placental function and subsequent adverse offspring outcomes can be attributed to several key mechanisms. Firstly, dysbiosis in the paternal gut microbiota can lead to alterations in the male reproductive system, including impaired leptin signaling. Leptin, a hormone involved in regulating energy balance, has been shown to play a crucial role in placental development and function. Disruption of leptin signaling due to dysbiosis can result in compromised placental health, leading to conditions such as in utero placental insufficiency.
Moreover, alterations in the testicular metabolite profiles of dysbiotic fathers can also impact placental function. Metabolites produced in the testes can influence sperm quality and function, as well as have systemic effects on the body. Changes in these metabolite profiles due to gut microbiome perturbations can affect sperm composition and function, ultimately impacting the health of the placenta and the developing fetus.
Additionally, the remapping of small RNA payloads in sperm as a response to induced dysbiosis in the male reproductive system can contribute to the adverse offspring outcomes. Small RNAs carried by sperm play a role in regulating gene expression in the developing embryo and placenta. Alterations in the small RNA content of sperm due to dysbiosis can lead to changes in gene expression patterns in the offspring, potentially affecting their growth and development.
How do the observed intergenerational effects compare to those seen in maternal microbiome disruptions, and what are the relative contributions of paternal and maternal microbiomes?
The observed intergenerational effects resulting from paternal gut microbiome perturbations differ from those seen in maternal microbiome disruptions in several ways. While both paternal and maternal microbiomes can influence offspring health, the mechanisms and pathways through which they exert their effects can vary. Maternal microbiome disruptions are often associated with direct transmission of microbes to the offspring during pregnancy and childbirth, impacting the initial colonization of the infant gut microbiota and immune system development.
In contrast, paternal microbiome perturbations primarily affect offspring health through alterations in sperm composition and function, leading to changes in gene expression patterns in the developing embryo and placenta. The relative contributions of paternal and maternal microbiomes to offspring health can vary depending on the specific context and the nature of the perturbations. In the case of the gut-germline axis, paternal microbiome perturbations appear to have a significant impact on placental function and offspring outcomes, highlighting the importance of considering both parental contributions to intergenerational health.
Could targeted modulation of the gut-germline axis be a potential therapeutic strategy for improving intergenerational health outcomes?
Targeted modulation of the gut-germline axis holds promise as a potential therapeutic strategy for improving intergenerational health outcomes. By understanding the intricate relationship between the gut microbiota, the male reproductive system, and offspring health, interventions can be developed to restore microbial balance and optimize paternal health before conception. Restoring the paternal microbiota through probiotics, prebiotics, or fecal microbiota transplantation could potentially mitigate the adverse effects of dysbiosis on placental function and offspring outcomes.
Furthermore, interventions aimed at improving the overall health of the gut-germline axis, such as dietary modifications, lifestyle changes, and microbial therapies, could help prevent intergenerational health risks associated with paternal microbiome perturbations. By targeting specific pathways involved in the gut-germline axis, such as leptin signaling, testicular metabolite profiles, and small RNA payloads in sperm, therapeutic strategies can be tailored to address the underlying mechanisms driving adverse offspring outcomes. Overall, targeted modulation of the gut-germline axis represents a promising avenue for improving intergenerational health outcomes and preventing the transmission of disease risk from one generation to the next.
0
