Periportal Macrophages Protect the Liver from Commensal-Driven Inflammation
Core Concepts
Periportal macrophages expressing the scavenger receptor Marco suppress inflammatory responses in the liver, protecting it from commensal-driven inflammation.
Abstract
The content discusses the role of periportal macrophages in regulating immune responses in the liver. Key insights:
The liver is a heterogeneous organ with distinct zones, including the periportal vein (PV) and pericentral vein zones, which differ in their immune responses.
Intravital imaging revealed that inflammatory responses are suppressed in PV zones, which contain a subset of immunosuppressive macrophages.
These macrophages express high levels of interleukin-10 and the scavenger receptor Marco, which sequesters pro-inflammatory molecules and suppresses immune responses.
The induction of these Marco+ immunosuppressive macrophages depends on the gut microbiota, specifically the bacterial family Odoribacteraceae and its metabolite isoallolithocholic acid.
Disruption of the intestinal barrier leads to inflammation in PV zones, which is exacerbated in the absence of Marco.
Chronic liver diseases like primary sclerosing cholangitis (PSC) and non-alcoholic steatohepatitis (NASH) show decreased numbers of Marco+ macrophages.
Functional ablation of Marco+ macrophages leads to PSC-like inflammatory phenotypes and exacerbates steatosis in NASH animal models.
The findings suggest that commensal bacteria-induced Marco+ immunosuppressive macrophages play a critical role in limiting excessive inflammation at the liver's gateway, and their failure promotes hepatic inflammatory disorders.
Periportal macrophages protect against commensal-driven liver inflammation - Nature
Stats
The liver is the main gateway from the gut, and the unidirectional sinusoidal flow from portal to central veins constitutes heterogenous zones, including the periportal vein (PV) and the pericentral vein zones.
Induction of Marco+ immunosuppressive macrophages depended on gut microbiota, particularly the bacterial family Odoribacteraceae and its postbiotic isoallolithocholic acid.
Intestinal barrier leakage resulted in inflammation in PV zones, which was markedly augmented in Marco-deficient conditions.
Chronic liver inflammatory diseases such as primary sclerosing cholangitis (PSC) and non-alcoholic steatohepatitis (NASH) showed decreased numbers of Marco+ macrophages.
Quotes
"Functional ablation of Marco+ macrophages led to PSC-like inflammatory phenotypes related to colitis and exacerbated steatosis in NASH in animal experimental models."
"Collectively, commensal bacteria induce Marco+ immunosuppressive macrophages, which consequently limit excessive inflammation at the gateway of the liver. Failure of this self-limiting system promotes hepatic inflammatory disorders such as PSC and NASH."
Key Insights Distilled From
by Yu Miyamoto,... at www.nature.com 04-24-2024
https://www.nature.com/articles/s41586-024-07372-6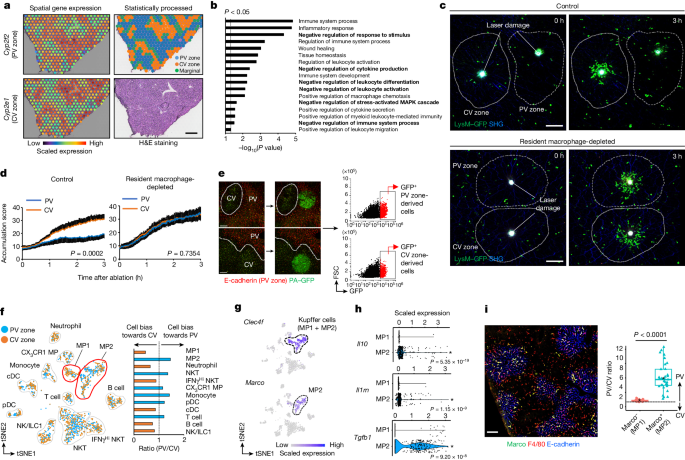
Deeper Inquiries
How do the immunosuppressive functions of periportal macrophages compare to other liver-resident immune cells, such as Kupffer cells or hepatic stellate cells?
The immunosuppressive functions of periportal macrophages, characterized by the expression of high levels of interleukin-10 and Marco, set them apart from other liver-resident immune cells like Kupffer cells or hepatic stellate cells. While Kupffer cells primarily act as liver-resident macrophages involved in phagocytosis and immune regulation, periportal macrophages exhibit a unique immunosuppressive phenotype that helps in limiting excessive inflammation at the gateway of the liver. On the other hand, hepatic stellate cells are known for their role in liver fibrosis and wound healing, distinct from the immunosuppressive functions of periportal macrophages. The specific expression of Marco by periportal macrophages allows them to sequester pro-inflammatory pathogen-associated molecular patterns and damage-associated molecular patterns, thereby suppressing immune responses and maintaining liver homeostasis.
What are the potential therapeutic implications of targeting the Marco+ macrophage population for the treatment of chronic liver diseases like PSC and NASH?
Targeting the Marco+ macrophage population presents promising therapeutic implications for the treatment of chronic liver diseases such as primary sclerosing cholangitis (PSC) and non-alcoholic steatohepatitis (NASH). Since Marco+ immunosuppressive macrophages play a crucial role in limiting inflammation at the periportal vein zones, their functional ablation or depletion could lead to PSC-like inflammatory phenotypes related to colitis and exacerbated steatosis in NASH. Therefore, therapeutic strategies aimed at enhancing the population of Marco+ macrophages or promoting their immunosuppressive functions could potentially alleviate liver inflammation and mitigate the progression of PSC and NASH. This could involve targeted drug delivery systems, immunomodulatory agents, or gene therapy approaches to specifically enhance the presence and activity of Marco+ macrophages in the liver.
Given the role of the gut microbiome in shaping the periportal macrophage population, how might dietary or probiotic interventions be leveraged to modulate this system and prevent or treat liver inflammation?
The influence of the gut microbiome on shaping the periportal macrophage population highlights the potential for dietary or probiotic interventions to modulate this system and prevent or treat liver inflammation. Dietary interventions that promote a healthy gut microbiome, such as high-fiber diets or prebiotic supplements, could help in maintaining the balance of commensal bacteria that induce Marco+ immunosuppressive macrophages. Probiotic supplements containing beneficial bacteria, especially those capable of producing postbiotic metabolites like isoallolithocholic acid, could also be leveraged to promote the generation of Marco+ macrophages and enhance their immunosuppressive functions. By modulating the gut microbiome through dietary or probiotic interventions, it may be possible to regulate the induction of immunosuppressive macrophages in the periportal vein zones, thereby preventing excessive inflammation and mitigating the development of liver inflammatory disorders like PSC and NASH.
0
