Chronic Myeloid Leukemia Diagnosed in a 65-Year-Old Woman with Unintended Weight Loss and Fatigue
Core Concepts
A 65-year-old woman with unintended weight loss and fatigue was diagnosed with chronic-phase chronic myeloid leukemia (CML) after comprehensive evaluation, and was successfully treated with a tyrosine kinase inhibitor.
Abstract
The content describes the case of a 65-year-old woman who presented with concerns of recent unintended weight loss and fatigue. Upon further evaluation, she was found to have splenomegaly and an elevated white blood cell count with low leukocyte alkaline phosphatase (LAP) levels.
The differential diagnosis considered various disorders that can cause splenomegaly and leukocytosis, including autoimmune disorders, infections, and myeloproliferative disorders. After performing a peripheral blood smear and bone marrow biopsy, the patient was diagnosed with chronic-phase CML, which was confirmed by the presence of the Philadelphia (Ph1) chromosome and BCR::ABL1 transcripts.
The patient was treated with the tyrosine kinase inhibitor (TKI) imatinib as primary therapy, and her disease was monitored using quantitative reverse transcription polymerase chain reaction (RT-PCR) to measure BCR::ABL1 transcripts. She achieved positive early response indicators at 3 and 6 months, indicating a favorable prognosis.
The content also discusses the recommended initial evaluation and monitoring for CML patients, including the use of bone marrow cytogenetics, FISH, and RT-PCR on peripheral blood to detect and quantify BCR::ABL1 transcripts. The choice of first-line TKI therapy is based on risk stratification, patient characteristics, and comorbidities. The main treatment goal is to achieve a complete cytogenetic response and major molecular response, which are associated with improved long-term outcomes.
Don't Miss the Dx: Woman Has Unintended Weight Loss, Fatigue
Stats
The patient's white blood cell count was elevated (> 40,000 cells/µL) with low leukocyte alkaline phosphatase (LAP) levels.
Peripheral blood smear showed a leukoerythroblastic blood picture with 10% peripheral blast cells.
Bone marrow biopsy showed hypercellularity with expansion of myeloid and myeloid progenitor cells.
Cytogenetic studies confirmed the presence of the Philadelphia (Ph1) chromosome and BCR::ABL1 transcripts.
The patient's BCR::ABL1 transcripts were 6% positive at 3 months and 2% positive at 6 months, indicating a positive early response to imatinib therapy.
Quotes
"Quantitative RT-PCR is a molecular technique used to measure the amount of specific RNA in a sample. The results of this test can be expressed in different ways, such as the ratio of BCR-ABL1 transcripts to control."
"Achieving an early molecular response (≤ 10% BCR::ABL1 on the international scale) at 3 and 6 months post-initiation of TKI therapy is a strong indicator of favorable long-term progression-free survival and overall survival."
Key Insights Distilled From
by Adam at www.medscape.com 04-25-2024
https://www.medscape.com/viewarticle/dont-miss-dx-65-year-old-woman-unintended-weight-loss-and-2024a10007ox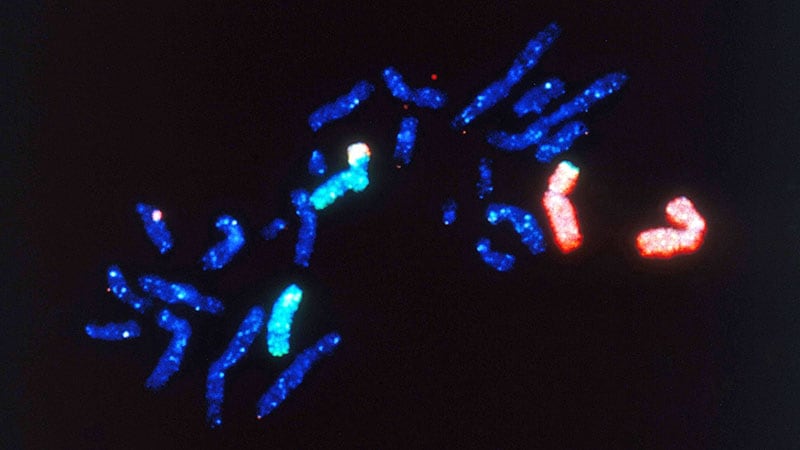
Deeper Inquiries
What are the potential long-term complications and side effects associated with chronic TKI therapy in CML patients?
Chronic TKI therapy in CML patients can lead to various long-term complications and side effects. Some of the common adverse effects include cardiovascular events like arterial occlusive disease, pulmonary hypertension, and QT prolongation. Other complications may involve hepatotoxicity, renal dysfunction, myelosuppression, and gastrointestinal disturbances such as diarrhea, nausea, and vomiting. Additionally, musculoskeletal symptoms like myalgia and arthralgia, as well as skin reactions, such as rash and dry skin, can occur. Long-term use of TKIs has also been associated with metabolic abnormalities like hyperglycemia, hyperlipidemia, and hypothyroidism. Regular monitoring and management of these side effects are crucial for optimizing the quality of life for CML patients undergoing TKI therapy.
How do the treatment approaches and outcomes differ for patients who develop resistance or are nonresponsive to multiple TKIs?
For patients who develop resistance or are nonresponsive to multiple TKIs, treatment approaches and outcomes can significantly differ. In cases of resistance, where the disease progresses despite TKI therapy, alternative treatment options need to be considered. This may involve switching to a different TKI with a distinct mechanism of action, such as second- or third-generation TKIs like dasatinib, nilotinib, bosutinib, ponatinib, or other investigational agents. In some instances, patients may benefit from combination therapies or experimental treatments through clinical trials. The prognosis for patients with resistance can be challenging, and close monitoring of disease progression is essential to guide further management decisions.
What are the potential implications of the increasing incidence of BCR-ABL1 transcripts detected in normal individuals as they age, and how might this affect the interpretation of molecular monitoring in CML?
The increasing incidence of BCR-ABL1 transcripts detected in normal individuals as they age poses potential implications for the interpretation of molecular monitoring in CML. As the incidence of these transcripts rises with age, especially in individuals over 60, distinguishing between normal age-related changes and disease progression in CML patients becomes more complex. This can lead to challenges in accurately assessing treatment response and disease monitoring. Therefore, it is crucial to establish baseline levels of BCR-ABL1 transcripts in older individuals to differentiate between benign elevations and disease-related changes. Additionally, incorporating clinical context, patient history, and other diagnostic modalities can help in the accurate interpretation of molecular monitoring results in CML patients, particularly in the elderly population.
0
