Transition-Metal-Free Formation of C(sp3)–C(sp3) Bonds via Frustrated Ion Pairs
This research paper introduces a significant advancement in organic synthesis, specifically in the area of cross-electrophile coupling reactions. The authors present a novel method for forming C(sp3)–C(sp3) bonds, which are ubiquitous in organic molecules, without relying on traditional transition-metal catalysts or pre-activated substrates.
The key innovation lies in utilizing frustrated ion pairs, which facilitate a unique single-electron transfer mechanism. This approach enables the coupling of unactivated alkyl electrophiles, expanding the scope of cross-coupling reactions to include functional groups that were previously incompatible with existing methods.
The authors demonstrate the versatility of their method by successfully coupling various substrates with different functional groups. They also highlight the potential of this new reactivity pattern for developing other transformative reactions in organic synthesis.
The findings of this research have significant implications for the field of organic chemistry. This transition-metal-free approach offers a more sustainable and cost-effective alternative to traditional cross-coupling reactions. Moreover, the ability to couple unactivated alkyl electrophiles with diverse functional groups opens up new avenues for synthesizing complex organic molecules, potentially leading to the development of novel pharmaceuticals, materials, and other valuable compounds.
요약 맞춤 설정
AI로 다시 쓰기
인용 생성
소스 번역
다른 언어로
마인드맵 생성
소스 콘텐츠 기반
소스 방문
www.nature.com
Coupling of unactivated alkyl electrophiles using frustrated ion pairs - Nature
핵심 통찰 요약
by Sven Roedige... 게시일 www.nature.com 11-20-2024
https://www.nature.com/articles/s41586-024-08195-1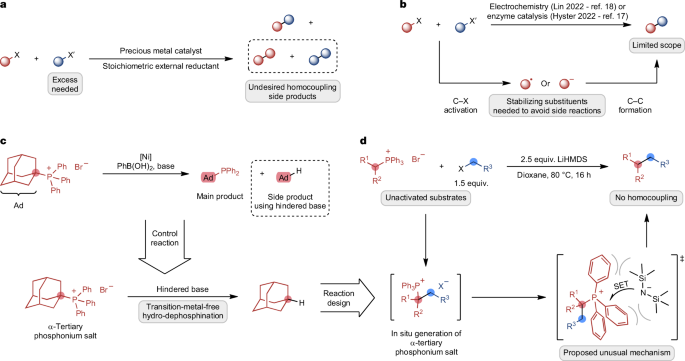
더 깊은 질문
